Introduction
Heat in an object does not remain stationary and tends to move from high-temperature to low-temperature areas. Thus, overheated materials tend to cool down while cold materials tend to absorb heat from the environment and warm up. Similarly, when two objects at different temperatures come into contact, heat energy is transferred from the hotter object to the cooler one. You might have seen dogs sticking out their tongues while panting. This helps them cool down because when they inhale, moisture in the air condenses on their tongues, forming liquid droplets that later evaporate. This evaporation requires thermal energy, which is supplied by their tongues, aiding them in cooling down.

Evaporation

Dogs cool themselves
What is conduction?
Conduction refers to the transfer of heat, electricity, or energy between particles of a substance without movement of the particles across regions of different temperatures themselves. It occurs in solids, liquids, and gases, and is the primary source of heat transfer inside solids. Since the atoms inside metals are placed close together, they conduct electricity well. Physically, conduction occurs by vibration. When a solid object is heated, its atoms/molecules start vibrating rapidly. However, since each atom or molecule is bonded to the next one, this vibration causes the neighbouring atoms to vibrate, and the process continues till the heat energy has spread across the whole solid.
Mode of conduction
Conduction occurs when there is a difference in temperatures across different portions of a material. Metals conduct heat very well whereas, liquids and solids tend to make bad conductors of heat. Furthermore, heat conduction is better when the surface area of the solid is large.
Thermal Conductivity
Thermal conductivity measures an object’s ability to transmit heat. It refers to the rate of heat transfer per unit time between the two ends of an object, given a specific cross-sectional area. We know that heat always flows from a higher temperature region to a lower temperature region. Suppose we were given a uniform shaft with length ‘l’ and cross-sectional area A. If the temperature at the two ends of the shaft were ,
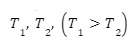
, and Q amount of heat was transferred through the shaft, then it is natural that Q would be proportional to the temperature difference, the cross-sectional area, and the time taken for the conduction to occur. Further, it would be inversely proportional to the length of the shaft. Hence, we can say that
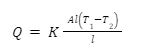
Here, K is the constant of proportionality known as the thermal conductivity.
What is convection?
Convection refers to the transfer of heat by the movement of particles in a medium from the hotter region to the colder region. It is a common way of heat transfer in liquids and gases. For instance, in a hot air balloon, the air molecules at the bottom of the balloon heat up and rise, creating low-density hot air that fills the balloon and causes it to ascend. Meanwhile, the cold air at the top of the balloon moves downward as the hot air rises, thus creating a continuous cycle of air movement.

Hot air balloons
Mode of Convection
- Chimneys are placed above stoves since hot molecules from our cooking will rise up and reach it via convection.
- Land and sea breeze flow based on convection. The land gets warmer faster than the sea, causing air molecules above it to rise up. The cool air from the sea flows in to maintain a pressure balance. The reverse process occurs at night.
What is radiation
Radiation is the transfer of heat without the need for particles or a medium to carry it. For instance, thermal radiation is how the sun transfers heat to the earth. During the process of radiation, heat is emitted from hot objects in all directions and unlike conduction and convection, radiation can take place even in a vacuum since it doesn’t require any a medium. Radiation of heat occurs in the form of electromagnetic radiation. Hot objects emit radiation in all directions, which is responsible for carrying heat and allows the heat energy to reach different places without the need of a medium.
Examples of conduction, convection, and radiation
- Metals are excellent conductors of heat, which makes them perfect for cooking. On the other hand, since wool is a bad conductor, we use it in our sweaters, which keeps the heat in.
- Thermometers utilise mercury, which is also an excellent conductor.
- Hot air balloons use the process of convection to function as described earlier.
- We are advised to use bright colours in summers since they reflect the heat energy coming to us in the form of radiation. Similarly, aircrafts are made up of bright colours so that they reflect whatever heat energy is incident on them.
Summary
Heat can be transferred between objects of different temperatures through convection, conduction, and radiation. Conduction occurs in solids while convection occurs in liquids and gases. Radiation can occur without the need for a medium. An example of convection is that of sea breeze and land breeze. During the day, land surfaces become warmer than seawater, causing the hot air to rise and cooler air to move towards the land at night. Temperature can be measured using Fahrenheit, Celsius, and Kelvin scales. The amount of heat energy in an object depends on the material’s mass, the temperature difference, and the material’s properties.
Frequently Asked Questions
1. What is Widemann-Franz Law?
This law states that the thermal and electrical conductivities of metals are directly proportional to their temperature, and at a specific temperature, they are equal.
2. What is Black body radiation?
A black body is a material that absorbs all radiation incident upon it. When held at a constant temperature, it emits all of the absorbed energy. The emitted radiation is independent of the material’s properties and is called black body radiation and it is emitted uniformly in all directions.
3. What is Stefan-Boltzmann law?
This law states that the heat energy emitted from a perfectly black body is proportional to the fourth power of temperature.
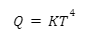
4. What happens when an ice cube is placed in water?
The process of conduction takes place when an ice cube is immersed in water. Heat from the water flows into the ice cube and melts it.
5. Discuss cooling via evaporation
Evaporation is a process wherein a liquid substance absorbs heat and changes into a gaseous state. Since heat energy is required for this to occur, evaporation can take away excess heat from objects and cool them down.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


