Introduction
Evaporation is the transition from the liquid to the vapour phase that takes place at pressures and temperatures below the boiling point (a condition of a substance just below its critical temperature). Evaporation occurs only when a substance’s relative vapour pressure is less than its equilibrium state vapour pressure. However, rather than a phase shift from liquid to gaseous, boiling is the formation of vapour as vapour bubbles immediately below the surface of the liquid. Rather than the literal conversion of the substance to gas, the term “vaporisation” has been used informally or hyperbolically to represent the actual physical disintegration of an object when subjected to high temperature or explosive force.
What is Vaporization?
Vaporization can be thought of as the transformation from a liquid to a gaseous state. When the temperature is raised, the kinetic potential of the molecules also increases. The force of attraction between molecules weakens as their kinetic energy increases. Because of this, they become airborne and spread over the area. This process requires the use of thermal energy.
Types of Vaporization
There are three types of vaporisation:
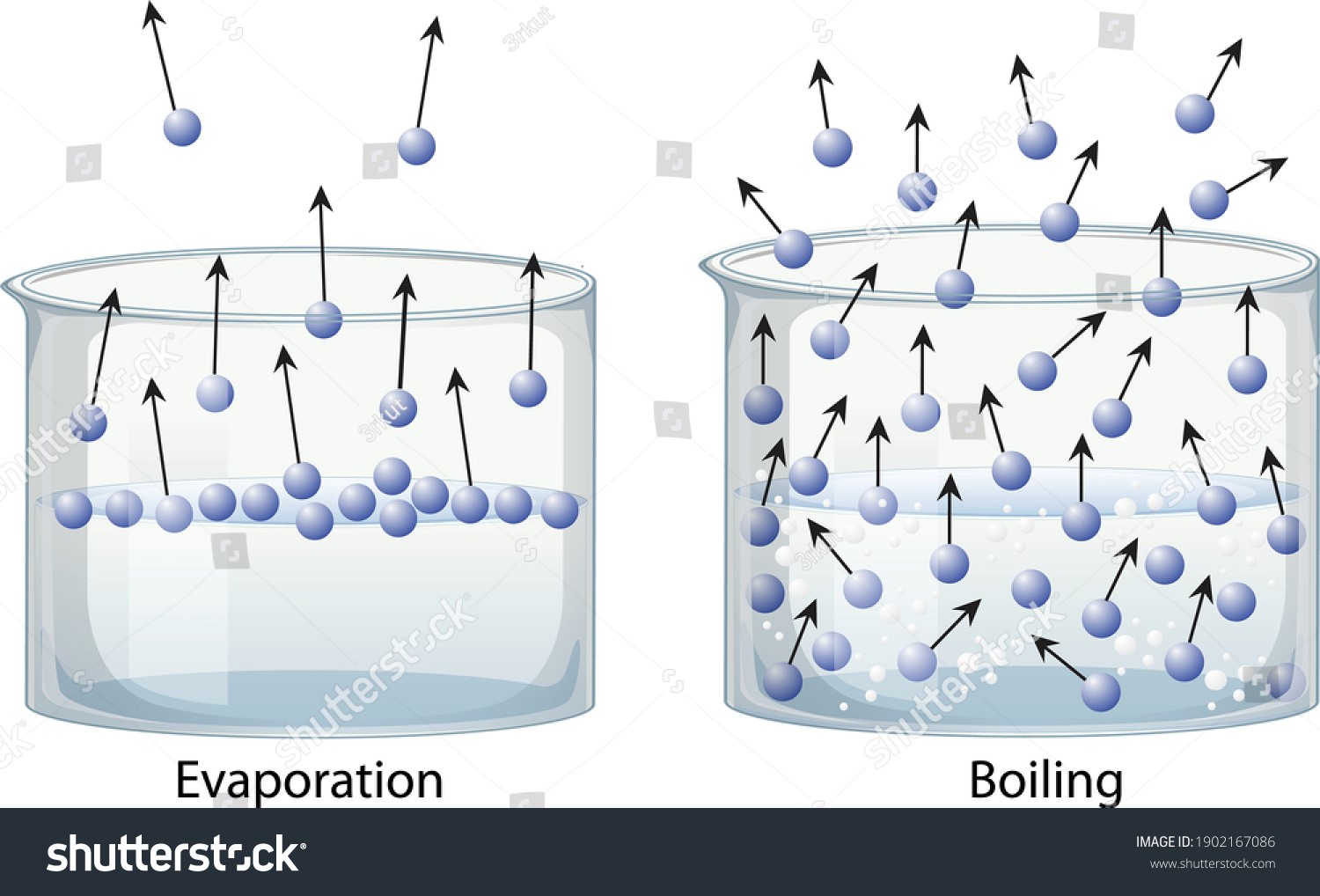
Evaporation
If you lower the temperature of a liquid below its critical point, you can cause a phase transition is known as evaporation to occur, in which the liquid changes into a gas.
The top is the primary site of evaporation. To begin evaporating, a substance must have a partial vapour pressure less than its equilibrium. So, for instance, if you continuously sucked the air out of a solution, you’d eventually be left with just a cryogenic liquid.

Boiling
Boiling is not a phase transition from the liquid state to the gaseous state but rather the creation of vapour below the surface of the liquid as vapour bubbles. Boiling occurs when the chemical’s equilibrium vapour pressure is higher than or equal to the ambient pressure. The boiling point of a substance is the temperature at which boiling occurs. The boiling point is affected by atmospheric pressure.
Sublimation
We know that ice melts into a liquid, and subsequently, the liquid evaporates into steam. However, there is a process through which matter can transition from its solid state into its vapour state, bypassing the liquid state entirely. Sublimation is the direct transformation of a solid into a gas.
Factors affecting the Rate of Vaporization
The rate of vaporization is affected by several factors, such as:
- The concentration of minerals in the solution.
- The amount of a substance that evaporates into the air.
- Due to the increased interactions between the molecules, more energy is needed to escape the liquid state.
- The point at which the liquid or gas begins to evaporate is called the vaporisation temperature.
- Reduced surface tension allows molecules to escape the surface faster, leading to increased evaporation rates.
- Evaporation rate is proportional to the surface kinetic potential of the molecules, which increases with temperature.
- Width of the Surface: Evaporation rates are proportional to the number of particles on the surface, so a bigger surface area means higher evaporation.
- If “clean” air (wind that is not yet laden with a drug or even other chemicals) is constantly passing across the substance, allowing for rapid evaporation, the amount of the chemical in the atmosphere is less likely to rise over time.
- Humidity refers to the amount of water vapour in the air.
- Because warmer air can hold more water vapour than cooler air, the evaporation rate will increase if the wind speed and humidity stay constant.
Examples of Vaporization in Our Daily Life
- Clothing that has been soaking wet can be dried through evaporation.
- This occurs when moisture in damp garments is evaporated when exposed to the sun’s thermal radiation.
- Separating the components of a mixture using this method is a common practice in many industrial processes.
- Using a vaporisation process, salt is produced from seawater in an industrial setting.
- Evaporation is used to remove salt from saltwater to produce table salt.
Frequently Asked Questions
1. What is the significance of the latent heat of vaporisation?
Ans: During a change of condition, heat energy is effectively hidden.
Latent heat, a form of hidden power used only during phase transitions, is also called the latent heat of vaporisation when it happens during the phase transition from liquid to gas.
2. What is critical temperature?
Ans. It is possible to define the critical temperature of a substance as the greatest temperature at which the substance can be in a liquid state.
No amount of pressure can cause a gas to turn into a liquid after it has reached a temperature over its critical temperature.
3. Does latent heat of vaporisation depend on the mass of the substance?
Ans. No, the latent heat of vaporisation does not depend on the mass of the substance. It has a fixed value at a given temperature and is not affected by the substance’s mass or volume.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


