Introduction
We can see the effects of oxidation and reduction reactions in daily life. This has a variety of consequences. Some of its instances, such as burning fuels, digestion of food in our bodies, and so on, are boons to humanity and highly beneficial to the continuation of life.
Do you know, in human bodies, respiration is the oxidation reaction? During this process, the food is oxidized and produces energy. On the other hand, some of its effects are highly harmful, such as air pollution from burning fuels, food rancidification, metal corrosion, etc.
Oxidation Reaction Examples
In many ways, oxidation reactions have an impact on our daily lives. While some of them are advantageous, others have unfavourable effects. The following are some typical oxidation reaction examples:
- In human bodies, respiration triggers an oxidation reaction. During respiration, food is oxidized to produce energy.
- Metal corrosion is a type of oxidation reaction.
- Fried foods acquire a bad flavour and a bad odour after being exposed to air for a long time (rancidity).
- Any substance that burns or is consumed undergoes an oxidation reaction, which always results in the production of energy.
- Energy is produced by the combustion of various fuels in a variety of domestic and industrial processes.
Oxidation Reaction’s Effects on Daily Life
Now let us discuss oxidation reactions in everyday life. Have you ever noticed how oxidation processes affect your daily life? Maybe you have, but you’re not aware that they involve an oxidation process. Rusting is an example of an oxidation reaction that you may be familiar with:
Rusting
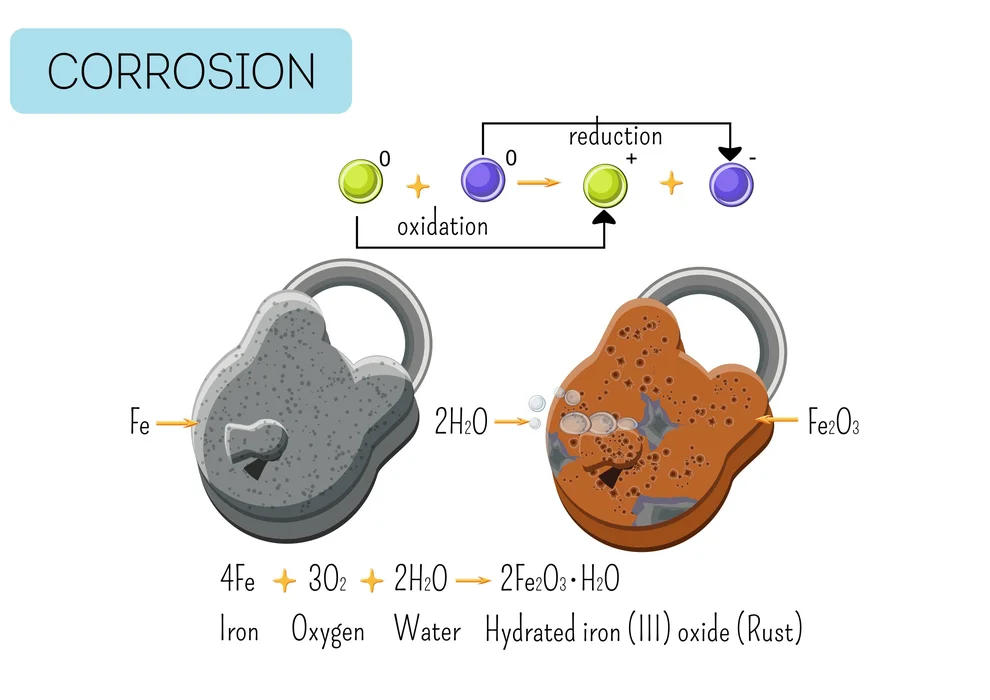
A type of metal corrosion is rusting. When air and moisture in the surrounding environment interact with a metal, corrosion results. It is a result of the metal oxidizing. Because iron oxidizes in the presence of air and water to produce hydrated iron oxide, it rusts (\(F{e_2}{O_3}.x{H_2}O\)). The metal surface develops a reddish-brown layer of iron oxide.
\[4Fe{\rm{ }} + {\rm{ }}3{O_2}\, + {\rm{ }}2x{H_2}O \to F{e_2}{O_3}.x{H_2}O{\rm{ }}\left( {Rust} \right)\]
Long-term corrosion or rusting harms metal-bodied constructions. Rust develops on car bodies, bridges, railings made of iron, and ships. The metal can be kept from corroding by having paint or enamel applied to its surface.
Rancidity
The rotting of food is another typical consequence of oxidation in daily life. When foods with fats or oils are left out in the air for a long time, they begin to rancid. This is caused by the food’s fatty acids slowly oxidizing in the air, which leaves the food tasting and smelling unpleasant. The phenomenon known as “rancidity” occurs when food items are exposed to the air and undergo colour, texture, taste, and odour changes due to atmospheric oxidation.

Combustion
One of the most significant oxidation reactions is combustion. Since energy is a by-product of all combustion reactions, these processes are known as exothermic reactions because they emit heat energy.
- Energy is a necessity for our society. Any fuel that burns in the presence of air, including kerosene, petroleum, coal, wood, and charcoal, produces heat. Methane in natural gas is burned during combustion, releasing carbon dioxide and water when there is too much oxygen.
- Thermal power plants burn coal to create electricity, while natural gas is used in kitchens. We can observe how important redox reactions are to maintaining our quality of life in this way. Fuel combustion generates thermal energy, which not only powers our economy but also keeps us warm and alive.
\[C{H_4}\, + \,2{O_2} \to C{O_2} + {\rm{ }}2{H_2}O\]
- Animals need the heat energy that they produced during food digestion. The human body also acts as a machine that burns and oxidizes all the food that is given to it to produce energy. The body gets its energy from sugar or carbohydrates like glucose (C6H12O6), fructose, and starch. When sugar and oxygen are burned, carbon dioxide, water, and heat are produced.
\[{C_6}{H_{12}}{O_6} + {\rm{ }}6{O_2} \to {\rm{ }}6C{O_2} + {\rm{ }}6{H_2}O{\rm{ }} + {\rm{ }}energy\]
The harmful effect of combustion
Despite the many benefits of combustion, its negative impact on our life needs to be properly addressed. Fossil fuel combustion results in hazardous vapours that contain dangerous gases such as carbon monoxide, nitrogen oxides, sulphur dioxide, and sulphur trioxide. When released into the atmosphere, fumes and smoke from furnaces and car exhaust severely pollute the air. It degrades our health and does direct harm to our bodies.
Summary
In addition to harming food, oxidation also harms metals. Corrosion is the term used to describe the harmful effect of oxidation on metals, and rancidity is used to describe it on food. Thus, the corrosion of metals and the rancidity of food are two common outcomes of oxidation reactions that are seen in daily life. Aerial oxidation is another name for the oxidation that oxygen in the air causes. The prevention of rancidity, corrosion, and their effects on daily life were all covered in this article.
Frequently Asked Questions
1.What happens when something oxidizes?
Ans. The deterioration in the quality of food products, including off flavours and odours, is caused by oxidation, a chain reaction that takes place in the presence of oxygen. It depends on how the product is made, how it is packaged, how it is stored, and what ingredients are used.
2. What distinguishes burning from combustion?
Ans. Combustion is just an oxidation reaction that releases energy; burning is a type of combustion that is followed by the evolution of gas and is distinguished by flame. Burning is combustion that results in a fire, but not all combustions result in a flame.
3. How is oxygen transported to the cell, so it can keep breathing?
Ans. Humans breathe in oxygen, which travels via many alveoli in the lung (tiny air sacs). These air sacs transfer oxygen into the blood, which carries it to the cells. The oxygen from the lungs is transferred to the blood, where it connects with the red blood cell’s haemoglobin and travels to all the cells where it is discharged. The lungs receive the waste carbon dioxide from the cells and transfer it there for expiration.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.



