Introduction
The valency of an element is indicated by its maximum capacity of making covalent bonds with any other element or the same element. Carbon has tetra valency since it can form a maximum of four bonds with another C atom or with other atoms like S, H, O, Cl, N, etc. Based on this, C atoms can form several organic compounds like Methane (\(C{H_4}\)), ethane(\({C_2}{H_6}\)), etc. Moreover, carbon can create compounds with both double and triple bonds among its atoms. Carbon chains can sometimes be ring-shaped, branched, or linear.
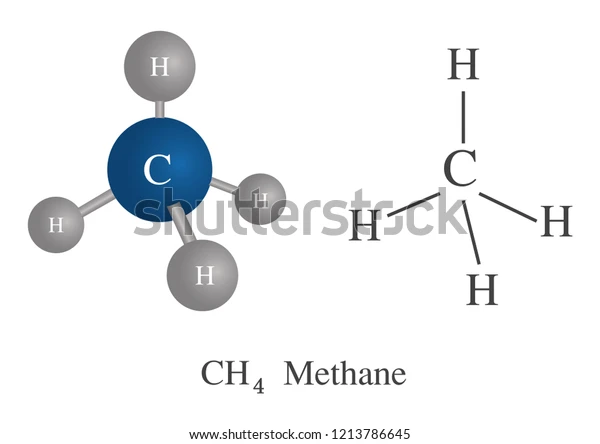
Define tetravalency of Carbon:
- Tetra signifies “four,” but “Valency” refers to “combining ability.” When carbon has a valency of four and can establish four covalent links with another atom, it is said to be tetravalent.
- The capability of carbon to form covalent linkages with the other carbon molecules tends to be referred to as catenation
- Due to the very small size of carbon, it can undergo catenation.
Reasons behind the tetravalency of Carbon:
The atomic number of carbon is 6 and its electronic configuration is \([He]2{s^2}2{p^2}\). The four electrons in the valence cells mean that the carbon atom can no longer lose or gain four electrons since doing so would require a significant amount of energy. Thus, the four electrons of carbon are shared with other elements. Since its electron has also been shared, there appear to be four shared electrons.
As a consequence, carbon’s valency becomes four, and as an outcome, “Carbon is Tetravalent.” Electrons can neither be generated nor taken up by carbon; it could only transfer them. Its tetravalent character influences the majority of the organic compounds.
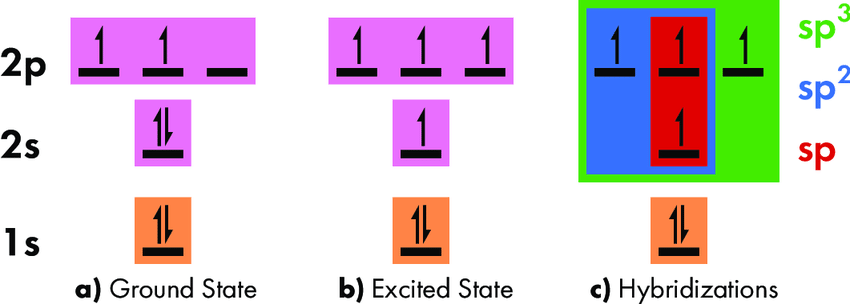
Explanation of tetravalency by ground state and excited state configuration of carbon:
tetravalency at Ground state:
The lowest energy level is called the ground state. Most of the electrons in carbon’s ground state are at their lowest available energies. Despite having four electrons, carbon can only create two bonds because its ground state only has two unpaired electrons.
Excited state:
Carbon is in an excited singlet state whenever the whole energy of the electrons may be lowered by first transferring one electron from the 2s orbital to the 2p orbital. In its excited state, carbon possesses four unpaired electrons, which allows it to form bonds with four other atoms.
For example, one 2s and three 2p electrons combine to form \(s{p^3}\) hybridization. Methane that is \(C{H_4}\) possesses \(s{p^3}\) hybridization of the C atom. The structure of methane is tetrahedral where C is linked with four H atoms and forms covalent bonds.
The hybridization of carbon in different compounds:
- \(s{p^3}\) hybridization: C atom forms four covalent bonds with four H atoms in\(C{H_4}\). The hybridization of C in \(C{H_4}\) is \(s{p^3}\). \(C{H_4}\) has a tetrahedral structure.
- \(s{p^2}\) hybridization: In ethylene molecules, two C atoms are joined together by one sigma bond and one pi bond. The hybridization of C in \({C_2}{H_4}\) is \(s{p^2}\).
- sp hybridization: Two sigma bonds and two pi bonds are observed in the structure of \({C_2}{H_2}\) (acetylene). Since carbon typically forms two sigma bonds, two of its valence orbitals can combine to generate two orbitals that seem to be equivalent to one another.

Summary
We may simply conclude that carbon tends to have the closest noble gas configuration since it couples its four valence electrons with several other elements and forms four separate covalent bonds. Tetravalency is the terminology used for this. In a later experiment, carbon proves that it has tetravalency across all hydrocarbons. The following features of carbon can make it the most flexible element in the periodic table: catenation, tetravalency, and isomerism. The production of such a large number of combinations from carbon compounds can be credited to each of these packages. Because they do not form bonds, inorganic composites have a lower number than organic composites.
Frequently Asked Questions
1. What other element other than carbon has tetravalency?
Ans: Si also can form four covalent bonds with other elements. That is Si is also tetravalent. But due to the larger size of Si as compared to C, Si doesn’t participate in catenation. Also, Si can’t form various compounds as C atoms can.
2. Is tetravalency possible for all semiconductors?
Ans: There is only one kind of element in it. The most abundant intrinsic semiconductor elements are silicon (Si) and germanium (Ge). Four valence electrons make them tetravalent. At the temperature of absolute zero, a covalent bond connects them to the atom.
3. Why is carbon considered to be a weak conductor of electricity?
Ans: Carbon compounds are found to contain covalent bonds. Covalent molecules do not disintegrate into ions in the aqueous phase; hence they do not possess any free electrons. Even though there would be no charge transfer, this becomes a poor conductor of electricity.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


