An Introduction to Matter
In addition to taking on various forms, the matter is composed of small particles. Because they are so small, it is impossible to see these particles with the human eye. We have mentioned below some of the various properties of matter. There are different states of matter can also be found. The three common states are solids, liquids, and gases. Atoms and other particles with mass and volume are included in the matter.
What do you understand by the Characteristics of Particles of Matter?
We are aware that every substance in our environment is composed of small particles. This means that these particles have some attributes and can affect the status of properties. These characteristics of the substance can be either physical or chemical.
Why are the particles drawn to one another?
1. The intermolecular force of attraction is the mechanism that pulls the particles toward one another. Additionally, the strength fluctuates according to the substance’s state.
2. The strongest and most stable objects are solids because they have the most of this force.
3. Since gases are not rigid and can take the shape of a vessel and be squeezed, we can say that it is significantly less in gases.
4. A higher force of attraction exists between the molecules of solids than in liquids and gases. However, the intermolecular force of attraction in liquids is higher than that of gases, making them less compressible than the gases.
Particles of Matter Attract Each Other
Let us understand, this characteristic with the help of two different activities.
Activity-1
1. Take a coin and a chalk
2. On trying to hammer the chalk and coin, the chalk gets powdered with great ease. On the other hand, the coin does not break easily.
3. This shows that the coin has a relatively strong force of attraction, followed by chalk, which has the least force of attraction.

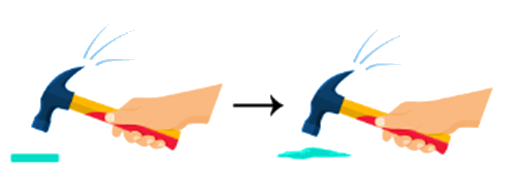
Activity-2
1. If we try to move our hands through water and air, our hands can move easily through the air as against the water.
2. This is because the force of attraction between the particles in water is greater than that in air.
Summary
Every particle of matter is always drawn to other nearby particles. The intermolecular force of attraction is the name given to this force of attraction between the component particles. The particles of matter are held together by this force. We can also conclude that solid has the greatest amount of force of attraction between particles, followed by liquids and gases have the least amount of force of attraction.
Frequently Asked Questions
1.Do matter’s constituent particles always attract one another?
Ans: Every particle of matter is always drawn to other nearby particles. The intermolecular force of attraction is the name given to this force of attraction between the component particles. The particles of matter are held together by this force.
2. What are the several forms that matter can take?
Ans: Solids, liquids, and gases are the three states in which matter can be found. Ice is a solid, water is a liquid, and steam is water in a gaseous state. Therefore, matter exists in all three states.
3. How can you ascertain the material’s physical characteristics?
Ans: We are aware that everything we see is made of something. They take up space and have mass. It’s crucial to realize that not all matter has the same physical characteristics. One common illustration of this fact is the fact that while sand particles are insoluble in water, salt particles are. Therefore, these elements can be referred to as matter’s physical characteristics.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


