Introduction
The ozone layer is like a savior for earth and mankind. It acts as a protective covering around the surface of the earth. It makes us safe from the ultraviolet (UV) radiation coming from the sun which directly hits the earth’s surface. The Ozone layer is situated at the end of the second layer of our earth’s atmosphere i.e. in the stratosphere. It gets damaged by various free radicals around us i.e. nitric oxide (NO), atomic bromine (Br), atomic chlorine (Cl), a hydroxyl group (OH), etc. These radical catalysts boost the depletion rate by many folds. Continuous damage to the ozone layer is causing serious health effects on the whole earth. As a result, UV radiation absorbing capacity is decreasing rapidly.
Define Ozone layer
It is a layer of protective covering formed by a molecule O3 around our earth’s atmosphere ranging from 15-30 km. This saves us from life-threatening harmful ultraviolet (UV) radiation emitted by the sun. The ozone (O3) can absorb that radiation and give us a safe surrounding to live in. Among several layers of the earth, the second layer i.e. stratosphere contains the maximum density of this ozone layer as compared to any other atmospheric layer of the earth. This discovery of this ozone layer was in the year of 1913 by two French physicists Charles Fabry and Henri Buisson. It is assumed to absorb 95-97 % of the medium and little high-frequency radiation coming from the sun.
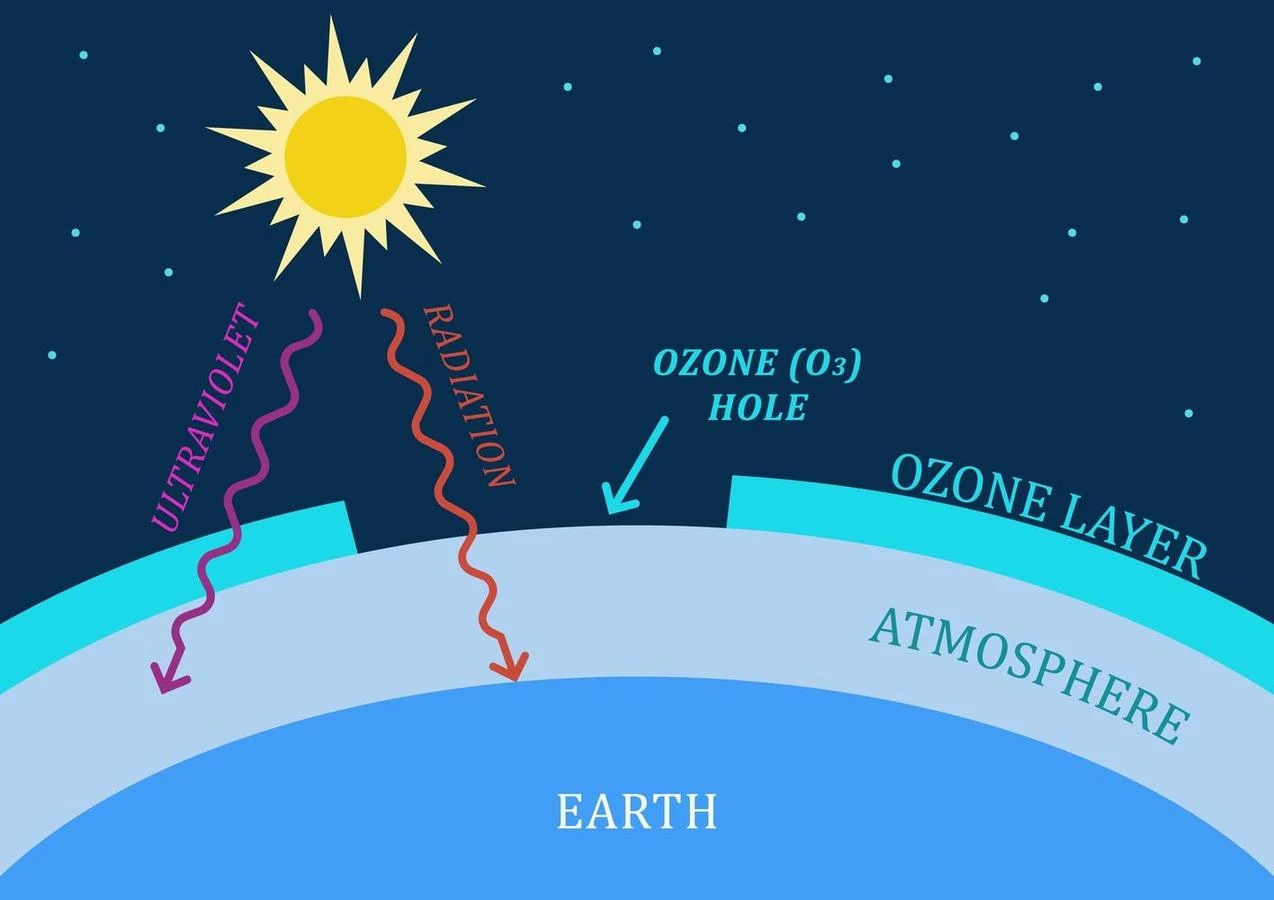
What is the depletion of the ozone layer?
Depletion of the ozone layer means degrading the layer formed by the ozone(O3) molecule around our atmosphere. This restricts the amount of harmful cosmic rays and radiation from the sun. Day by day the thickness of this layer is getting reduced and also holes are forming at many places due to the heavy damage of ozone molecules. It is more observed at both poles of the earth. Due to the irresponsible behavior of mankind, various chlorine (Cl) containing substances get released into the earth. They rise above and react with O3 and degrade it. It is also thought that one Cl atom can degrade around a lakhs of ozone (O3) molecules. Substances like chlorofluorocarbons (CFC) from refrigerators, carbon tetrachloride, hydrochlorofluorocarbons, etc. are highly responsible for such heavy damage.
Factors responsible for the depletion of the ozone layer
- Nitric oxide (NO), nitrous oxide (N2O), and nitrogen dioxide (NO2) are among the nitrogenous chemicals that have a significant impact on the ozone layer’s depletion or breakdown.
- Solvents, freezers, spray aerosols, air conditioners (A.C.), and other devices emit chlorofluorocarbons, which is one of the leading reasons for the ozone layer depletion represented by CFCs. Ultraviolet (UV) radiation in the stratosphere decomposes chlorofluorocarbon molecules, releasing chlorine (Cl) atoms in the process.
- The ozone layer molecules are also severely harmed by irregular or unmanaged rocket launches; in fact, these activities contribute more to the ozone layer’s destruction or depletion than CFCs do.
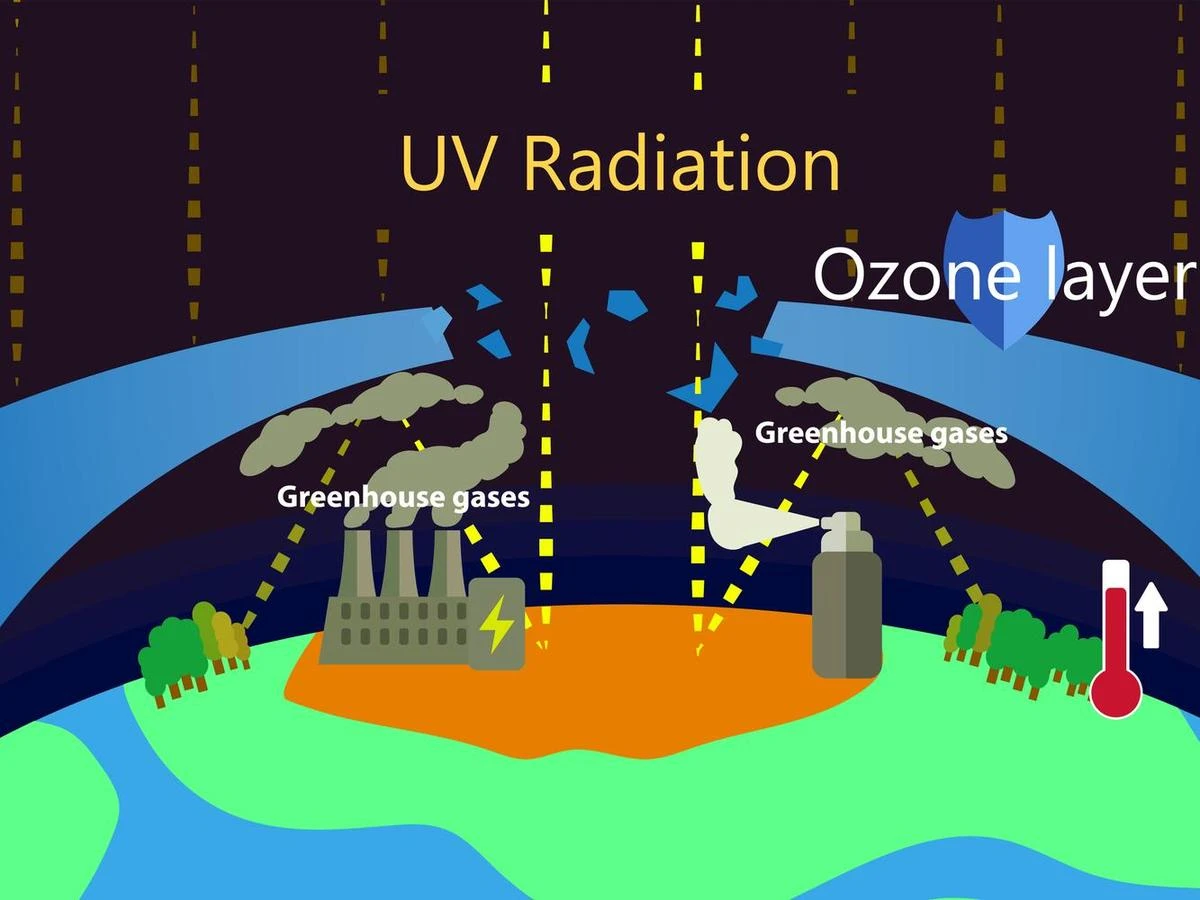
Effects of ozone layer depletion
The environment and all life forms that exist on the surface of the earth are seriously impacted by the breakdown of the ozone layer which increases the amount of radiation reaching the earth’s surface. Eye failure, various forms of skin cancer, and the body’s immune disorders are some of these adverse effects. Both aquatic ecosystem and terrestrial ecosystem also impacted by harmful UV radiation.
Effects of ozone layer depletion on mankind
Humans are adversely affected by the breakdown of the ozone layer, including the following effects:
- It is well understood, the loss of the ozone layer can result in more ultraviolet (UV) radiation reaching the earth’s surface, which enhances the danger of skin cancer and may also cause eye cataracts and immune system damage.
- Melanoma, the most fatal form of skin cancer, can increase as a result of excessive UV light exposure.

Effect of the damaged ozone layer on animals
A few harmful effects on animals are as follows:
- Fish, frogs, and other marine species’ early or beginning embryonic phases have been known to be harmed by ozone layer loss.
- Animals’ capability to breed slows down as ozone layer depletion occurs.
- Small fish or marine species may experience colony declines even with a slight increase in UV exposure.
Effects of ozone depletion on plants
- Plant developmental and physiological functions are strongly impacted by ultraviolet light.
- The growth, adaptability, and repair of flora are impacted by these dangerous radiations.
- These damaging radiations can inhibit the nutrient cycle and lead to plant diseases.
Summary
The area known as the earth’s stratosphere contains the ozone layer, also called the ozone screen. The majority or all of the ultraviolet (UV) rays from the sun are absorbed by this layer. Free radical catalysts can damage the ozone layer. In recent decades, there has been a marked rise in the emission of greater quantities of chlorine (Cl) and bromine (Br) into the atmosphere. This is due to the reckless discharge of vast amounts of chlorofluorocarbons (CFCs) and bromofluorocarbons by irresponsible human activities. All these are affecting human and animal life in a very adverse manner.
Frequently Asked Questions
1. How can the ozone layer be preserved?
Ans: Purchasing an air conditioner and refrigerator which doesn’t use HCFCs as a coolant. Also using perfumery products having no CFCs and HCFCs. It is mandatory to regularly check for any leakage in refrigerators and air conditioners.
2. Can humans survive in a world without ozone?
Ans: The shielding ozone, often known as the “ozone layer,” is essential to life. Radiation from the sun includes heat, light, and many other kinds of radiation. UV (ultraviolet) radiation exposure too much can injure both plants and animals as well as induce cataracts and skin cancer.
3. Where does ozone become hazardous to humans?
Ans: Ozone is a colorless substance that, depending on its location, may be beneficial or harmful. Because it protects the planet from the sun’s UV radiation, the ozone found in the stratosphere is beneficial. Because it could be harmful to human health at the surface level, where we inhale.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


