Introduction
In the modern periodic table, the elements are organized according to their atomic numbers. There are 7 rows and 18 columns in the periodic table. Noble gases belong to group 18 in the periodic table. The gases are helium, neon, argon, krypton, xenon, and radon. They remain in a gaseous state at normal pressure and temperature. To maintain inert conditions these noble gases have a great role.
Define Noble Gas
The noble gases have their valence shell completely occupied. Helium, neon, argon, krypton, xenon, and radon are known as “noble gas”. Due to their inertness, they react with very few elements. Even upon the addition of strong acids or bases, it remains unreacted. For this reason, they are named so.
Physical characteristics of noble gases
- Atomic size: On moving down the group, one more shell gets added up. That is why nuclear attraction towards the valence electrons decreases. So, atomic size goes on increasing.
- Melting point and boiling point: Due to very poor interatomic interaction, the melting point and boiling point of noble gases are very low as compared to other elements in the periodic table. Among them, helium has the lowest boiling point of -268.9 ℃. At room temperature, all these elements are in gaseous form.
- Density: Usually the density of these noble gases is very low. But on descending the group, density increases as mass increases. Radon has the highest density among them.
- Solubility: Noble gases dissolve in water at a very low extent. The dipole-dipole interaction is the main reason for their solubility in water. On moving down the group solubility increases.
Chemical properties of noble gases
- The valence shells of noble gases are completely occupied. So, they are inert.
- Noble gases are very stable and always stay in the ground state energy level.
- Only krypton, radon, and xenon can react chemically with high electronegative elements.
- Noble gases are inflammable.
- They have a very good electricity-conducting nature so they are also used as fluorescence material.
Application of Noble gases
Helium

- Due to its low density, helium is utilized to fill airships and colorful balloons.
- At -269℃, helium can boil. Due to its low boiling point, it functions as a very effective coolant. Electrical resistance is lost.
- Sea divers carry tanks with a mixture of helium and oxygen in them. The divers can breathe in a nitrogen-free environment as a result, and deep diving nitrogen narcosis is prevented.
- Superconducting wires are used in the coil of MRI machines to carry huge electric currents. Strong magnetic fields could form as a result of the electric currents. Helium assists in lowering the conducting coils’ temperature to the point where superconducting qualities may be seen.
Neon
- Neon used in vacuum discharge tubes receives electrical energy and glows as a result neon is utilized in displays.
- Lasers are made from neon and helium.

Argon
- For both incandescent and fluorescent bulbs, it is a crucial element. Because it won’t react with a tungsten coil, it is utilized in incandescent light bulbs instead of air.
- It provides an inert insulating condition for titanium fabrication where arc welding is used.
- It is used to create the conditions devoid of oxygen required to grow semiconductor crystals.
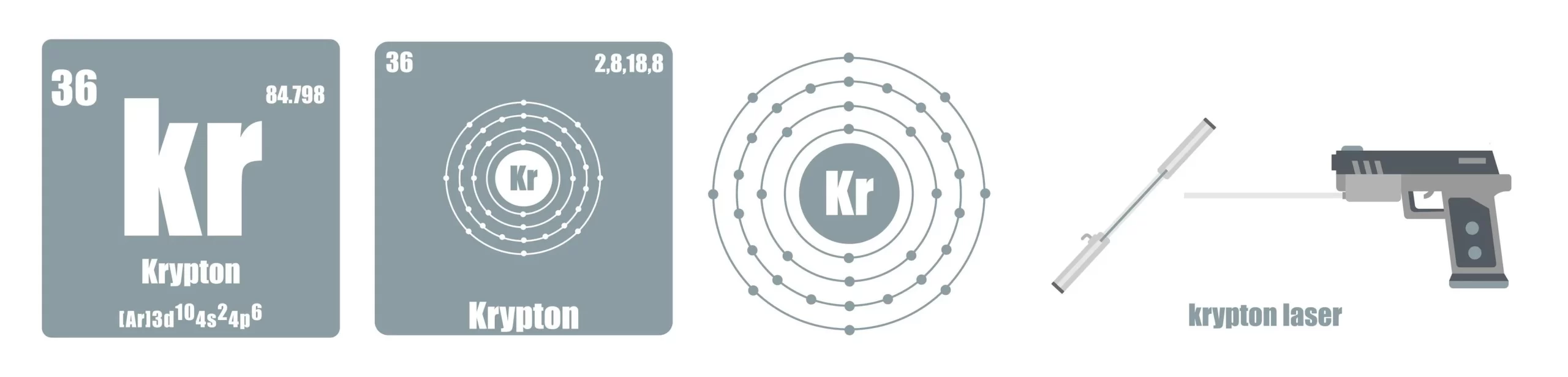
Krypton
- This gas has a very useful role in advertising signboards because it shines brightly when electricity is passed through krypton vapor inside a vacuum discharge tube.
- Krypton lasers are used during surgery to cure some eye conditions and to remove birthmarks.
- High-speed photography frequently uses argon and krypton to create photo flashes.
Xenon
- Bacteria-contaminated surfaces can be eliminated by the illumination produced by xenon lamps.
- It produces a bright white light that flashes, making it ideal for creating strobe lights.
- To increase the clarity in CT scan imaging, oxygen and xenon are combined.
Radon
- When it is dissolved in water, it can treat inflammatory disorders such as arthritis.
- Radon’s inherent radioactivity promotes cancer treatment. Direct placement of radon glass vials is one method of performing targeted radiation therapy.
Summary
The periodic table’s right-hand end column is where noble gases can be found. Helium, neon, argon, krypton, xenon, and radon are all non-metals and can also be referred to as inert or “noble gases”. The sizes of atoms of these gases increase as one descends from helium to radon. The density, melting, and boiling temperatures of the noble gases are extremely low, and they are insoluble in water. Noble gases are all odorless, colorless, and poor heat conductors. They have a very low reactivity to chemicals and are combustible.
Frequently Asked Questions
1. What distinguishes radon from other noble gases?
Ans. The heaviest noble gas, radon (Rn), is radioactive and occurs naturally as a byproduct of the disintegration of radium, thorium, and uranium. As a result, radon is unique among noble gases.
2. What occurs when an electric current travels through noble gases?
Ans: Because of their chemical inertness, noble gases are employed in displays. When an electric current is applied inside a vacuum discharge tube, they emit a dazzling glow.
3. What are the common characteristics of periodic groups?
Ans: A set of periodic tables’ elements share similar chemical characteristics that result from the existence of many electrons. It takes into account the number of valence electrons an atom has in its outer shell.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.



