Introduction
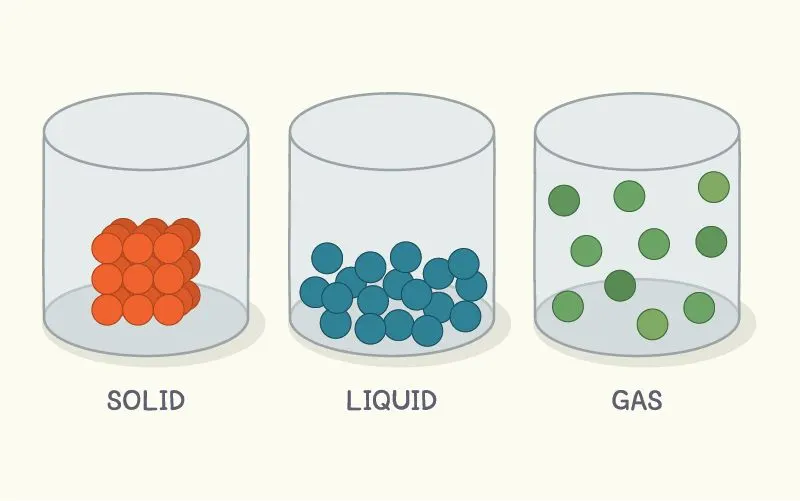
The different forms of \({H_2}O\) in three different phases are ice (solid), water (liquid), and gas (vapour). The intermolecular spaces in solid particles are very low, they are tightly bound to each other. The intermolecular spaces are comparatively higher in liquids and become maximum in vapours. With the increase in temperature, the kinetic energy of the molecule increases, and the intermolecular interaction between the particles decreases. That is why, on the application of heat, ice is transformed into water and then to vapour Melting and boiling depend on the pressure of the environment. On this basis, a pressure cooker is used to make food in daily life.
What is the melting point of ice?
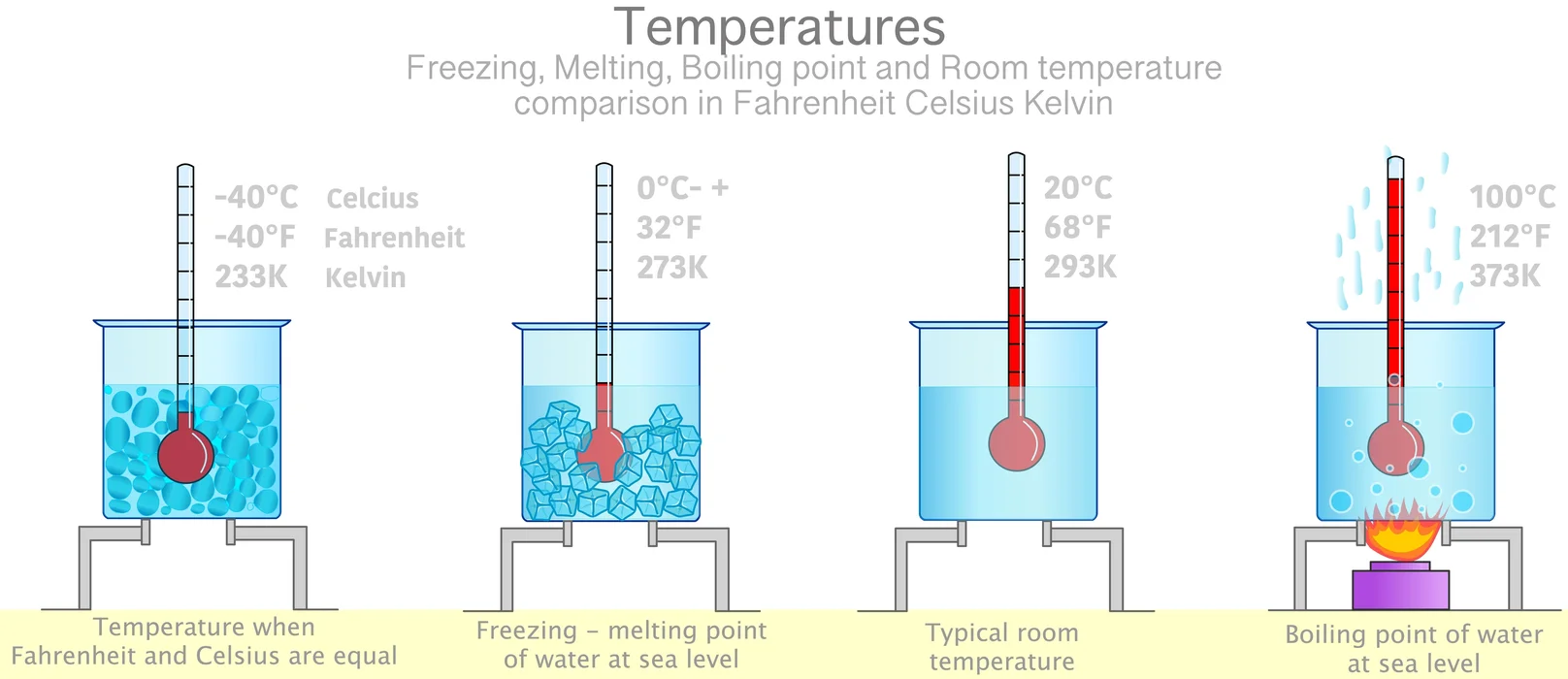
The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium, and the temperature at which matter changes from solid to liquid form. The term applies to pure liquids and solutions. The melting point depends on pressure, so it should be specified. This melting point of ice is 0℃. If the temperature increases beyond the melting point, it doesn’t increase the temperature of the matter. Rather, it helps to transform the ice completely into the water. This is known as the ‘latent heat of fusion’ of ice.
What is the boiling point of water?
With the addition of further heat, the water (liquid) reaches its vapour phase (gaseous state) at a particular temperature. This is the boiling point of water. The boiling point of water is 100℃. The heat that helps to convert the whole water into the gaseous state is known as the ‘latent heat of vaporisation’ of water.
Online Science Course for Classes 6th, 7th, and 8th. Clear your doubts from the Science experts teacher.
Method to determine the melting point of ice:
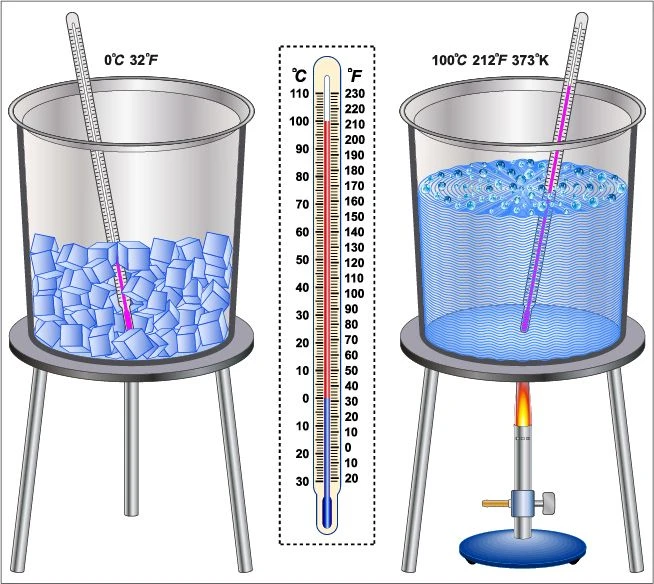
The melting point of ice is 0℃. It is determined in a laboratory in the following steps-
- Some ice cubes are taken in a beaker and a thermometer is dipped in it.
- Heat is applied gently by the Bunsen burner below the beaker.
- The changes in the state of the ice are monitored every minute and the temperature is recorded at which the whole ice is transformed into water.
- The temperature at which the ice starts melting is noted as \({t_1}\) and the temperature when all the ice melts is noted as \({t_2}\).
- Then the average of \({t_1}\) and \({t_2}\) is calculated. This mean temperature is known as the melting point of ice.
In this way, the melting point of ice is determined.
Method to determine the boiling point of water:
The water boils at 100℃. It is measured in the laboratory in the following steps-
- A suitable amount of water is taken in a round bottom flask and its mouth is sealed properly with a rubber cork.
- A thermometer is inserted through a hole in the cork into the r.b flask without touching the water surface.
- The r.b flask is then heated by a Bunsen burner, and placed on the bottom of a wire gauge supported by a tripod stand.
- A thermometer reading is taken at a certain interval and the temperatures are recorded.
- A thermometer reading is taken continuously throughout the boiling of water.
In such a way, the boiling point of water is measured.
Factors influencing the melting point and boiling point:
| Factors | Melting point | Boiling point |
| Pressure | With an increase in pressure, the melting point decreases. | With an increase in pressure, the boiling point increases. |
| Impurities | Impurities like soluble salts decrease the melting point. | The presence of impurities increases the boiling point. |
| Size of substance | With an increase in the size of the substance, the melting point increases. | With an increase in the size of thesubstance, van der Waals interaction increases henceboiling point increases. |
| Intermolecular forces | The melting point increases with an increase in intermolecular interactions, as more energy is needed for bond cleavage. | The stronger the intermolecular forces, the lower the vapour pressure. As a result, the boiling point increases. |
Summary:
The temperature at which ice starts to melt into water is called the melting point of ice (0℃). And the temperature at which water starts to form vapour is termed the boiling point of water (100℃). Intermolecular interaction decreases while moving from ice to water to gas. So, through the melting point of a solid and the boiling point of the liquid, one can have an idea about the extent of interaction among the particles. Moreover, the presence of impurities can be determined from the melting and boiling point values.
Frequently Asked Questions
1. Can the size of a molecule affect the melting point value?
Ans: With an increase in size, the van der Waals forces among the molecules increases. As the melting point highly depends on attractive forces i.e. van der Waals interaction, the size can impact the melting point value of the molecule.
2. Why is the boiling point of water always considered to be 100℃?
Ans: The liquid will start to boil once the vapour pressure of the liquid matches the atmospheric pressure in the region. The point at which water boils is highly influenced by the vapour pressure. At 100℃, the vapour pressure around sea level equals the surrounding air pressure. So, it is considered the boiling point of water.
3. How can one identify a substance by its melting point?
Ans: Various organic, as well as inorganic compounds, can be identified from their melting point. Also, the extent of purity can be known from their melting point values. If the substance is pure, it will show a sharp melting point instead of a range of melting points in case of an impure substance.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.



