Introduction
In the Friedel-Crafts reaction, an alkyl or acyl group swaps places with the hydrogen atom of an aromatic molecule to make a hydrocarbon or ketone. This is an example of an electrophilic substitution reaction. When an aromatic molecule is alkylated or acylated, an acid catalyst (Lewis acid) is present. This could be \(AlC{l_3}\), \(ZnC{l_3}\), or \(B{F_3}\). The attacking particle is an alkyl or acyl cation, which is made by the catalyst. In 1877 and 1878, J. Crafts and C. Friedel came up with this reaction. They used it to connect substituents to aromatic rings.
During the alkylation and acylation processes, the hydrogen atom that was originally attached to the aromatic ring is swapped for an electrophile. The most common catalyst is aluminium trichloride, which works as an acid (Lewis) by combining with chlorine to make a strong electrophile.
What is Friedel crafts reaction?
These reactions are organic coupling reactions that attach substituents to aromatic rings through an aromatic substitution that is electrophilic. During a typical Friedel–Crafts reaction, a new C–C bond is made. Friedel-Crafts processes can be either acylation or alkylation. A process called alkylation is used to add the benzene ring and a short carbon chain. When an acyl group is added in the process of acylation, aryl ketones are made.
What is its type
The two main categories of Friedel-Crafts processes are:
- Friedel craft acylation
- Friedel crafts alkylation.
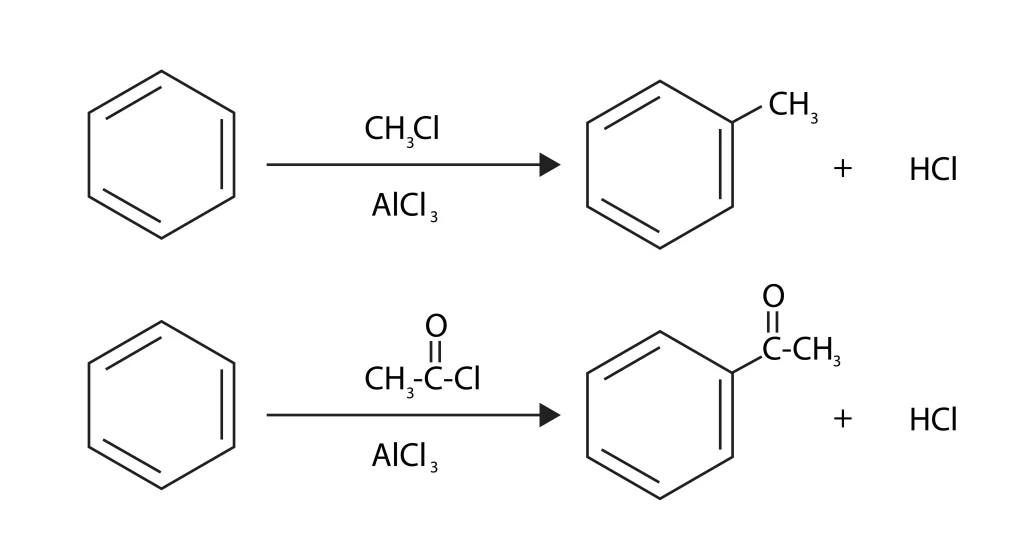
Friedel Craft Alkylation and Acylation
Friedel craft alkylation
When there is an aromatic ring, Friedel Crafts alkylation is done by using a Lewis acid on an alkyl halide. While the C-H bond is being broken, the alkyl group joins the ring and forms a C-C bond. A Lewis acid catalyst like \(FeC{l_3}\) or \(AlC{l_3}\) is used in this reaction to speed up the removal of the halide and make a carbocation. Before the alkylation reaction can start, the resultant carbocation has to change its shape. You should never use alkenyl, alkynyl, or aryl halides; only an alkyl halogen (Cl, Br, or I) should be used, or the reaction won’t work. The carbocations of these species are hard to make and very unstable.
This is the main way that the industry makes high-octane fuels, surfactants, fragrances, antioxidants, etc., as well as other important goods like cumene and thymol.
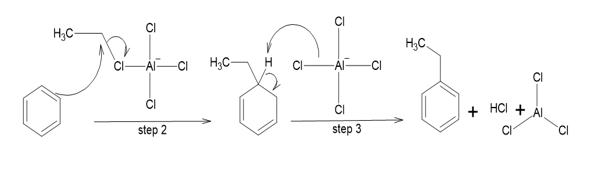
Mechanism of Friedel craft alkylation
STEP1: The lewis acid attavks the alkyl halide which results in the formation of a carbocation.
STEP2: The carbocation attacks the aromatic ring and an arenium ion is obtained.
STEP3: Deprotonation occurs at the ipso carbon and aromaticity the ring is restored and the alkylated aromatic molecule is obtained. The \(AlC{l_3}\) catalyst is renewed.
Friedel craft acylation
When a Lewis acid catalyst like \(AlC{l_3}\) is present, this aromatic electrophilic substitution reaction between arenes and acyl halogens or anhydrides and acyl halogens or anhydrides leads to the making of monoacylated compounds. The halogen in the acyl halide joins with the Lewis acid to form a complex. This makes a better electrophilic acylium cation (RCO+) that is stable through resonance. Ketones are the only thing that can come out of this process.
The Friedel Crafts acylation is an important business process. It is used to make fine chemicals, intermediates for making other chemicals, and chemical feedstock.
Mechanism of Friedel craft acylation
STEP1: The acyla halide and the lewis acid catalyst complexate. \(AlC{l^{4-}}\), is removed and an acylium ion is formed (RCO+).
STEP2: The acylium ion is an electrophile and it attacks the aromatic ring’ double bond resulting in the formation of an arenium ion.
STEP3: Deprotonation occurs at the ipso carbon and aromaticity the ring is restored and acylated aromatic molecule is obtained. The \(AlC{l_3}\), catalyst is renewed.
Summary
“Friedel-Crafts alkylation” is the process of replacing an aromatic proton with an alkyl group. To do this, a carbocation is used to attack the aromatic ring in an electrophilic way. Alkyl halides are used as reactants in the Friedel-Crafts alkylation method, which makes alkylbenzenes. During Friedel Crafts acylation, when an acyl group is added, aryl ketones are made.
Friedel Crafts acylation is better than Friedel Crafts alkylation in a number of ways. One of these benefits is the ability to better control the reaction products. Another is that the acylium cation is stable because of resonance, which makes rearrangement impossible. The Clemmensen reduction process can be used to turn the produced ketones into alkyl groups.
Frequently Asked Questions
1. Why can we not uses lewis base aromatic compounds in Friedel-Crafts reactions?
We can’t use Lewis base organic compounds because \(AlC{l_3}\), which is used in the Friedel-Crafts process and is a Lewis acid catalyst, reacts with Lewis base to make salt. Because the base gets a positive charge, it can be used to stop other reactions from happening.
2. Can Friedel craft reaction take place without catalyst?
No, Friedel craft reactions can not take place without catalysts. For the Friedel Craft reaction to occur without catalyst, the finest aromatic reagent are those that can produce the electrophile without needing Lewis acids. Since benzene rings are not strong nucleophiles, they can not produce electrophiles directly. Thus it needs an external help.
3. What is arenium ion?
An arenium ion is a cyclohexadienyl cation in organic chemistry. It is a reactive intermediate in electrophilic aromatic substitution. Two hydrogen atoms that are attached to a single carbon atom are in a plane that is perpendicular to the benzene ring. The arenium ion is no longer an aromatic species, but due to delocalization, it is still pretty stable:
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.



