Introduction
Distillation is an isolation technique used to separate two miscible or immiscible liquids. This is based on the principle of boiling points of the components of the liquid mixture. It has been used since ancient times to make oils, alcohol, beverages, etc. The distillation process has now been industrialised and made faster and more efficient with more delicate instrumentation.
What is fractional distillation?
Fractional distillation is another form of the distillation process that makes it simple to separate two or more liquids in combination with different boiling points but near one another. Vaporization is the method used for this separation. The process continues, with the liquid having a lower boiling point evaporating first and emerging from the mixture. With a pressure of 1 atm, the liquids typically have boiling points that are dissimilar by around 25 °C. Separation benefits greatly from fractional distillation. Thus, it is frequently used in a variety of sectors, including cryogenic air separation, chemical plants, oil refineries, and many others. Distillation typically operates at a constant state.
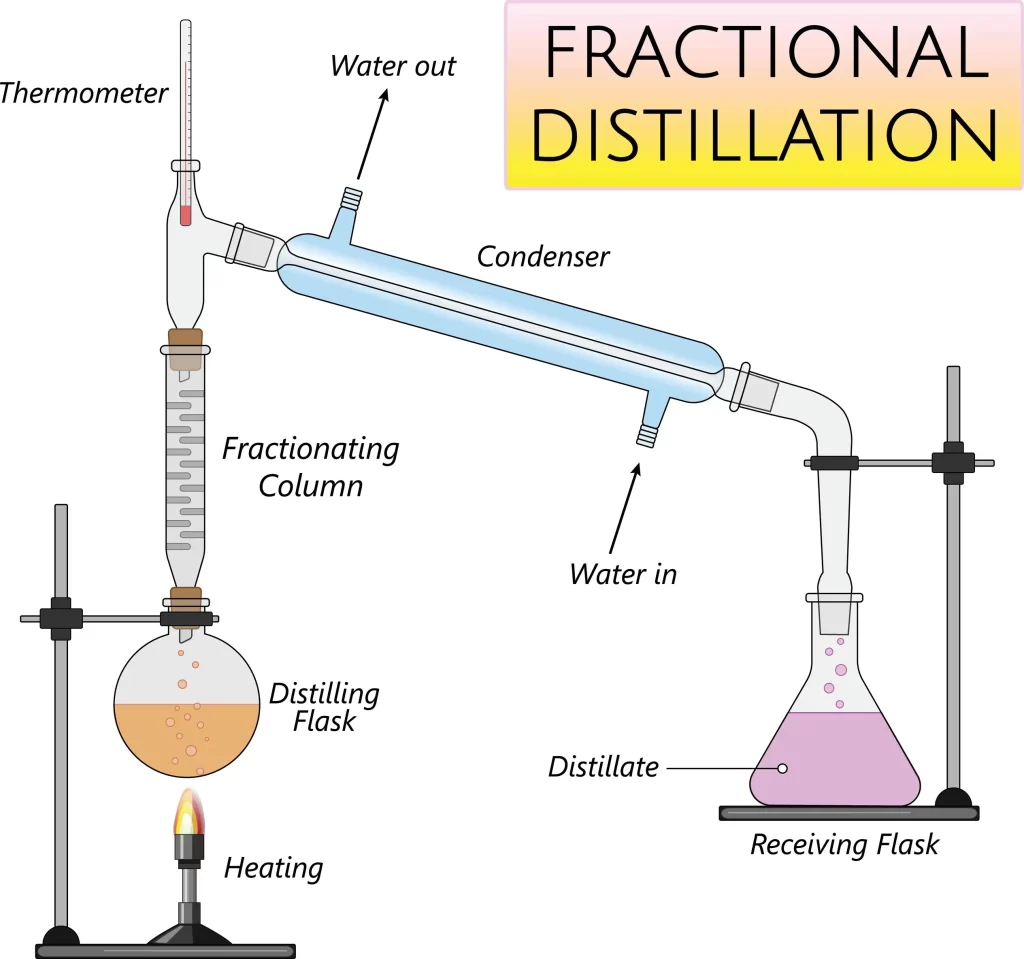
Fractional Distillation
Principle of fractional distillation
The difference in the boiling points (B.P.) of the mixture’s liquids serves as the foundation for the fractional distillation principle. The impurities contained there also exhibit distinct boiling temperatures when the same mixture is heated, making it simple to separate them using a pipe. Now, with the aid of an example or instance, we may comprehend this. One illustration is the fractional distillation of water and ethanol (C2H5OH). Considering ethanol’s boiling point is 78.5 °C and water’s boiling temperature is around 100 °C. As a result, the fractional distillation method may be used to separate the combination of these two liquids, as there is a sizable difference in their boiling points.
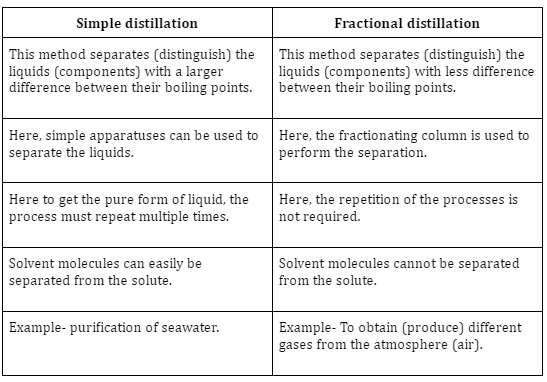
Difference between simple and fractional distillation
Fractional Distillation Apparatus
The fractionating column of the fractional distillation equipment increases its efficiency by giving the liquid more opportunities to condense or freeze, giving it an edge over a straightforward distillation procedure. In the fractional distillation process, a fractionating column packed with glass beads offers a large surface area for the vapours to clash and lose energy, allowing them to be condensed and distilled swiftly.
Fractional Distillation in industry.
The division of crude oil into its constituent parts is one of the most significant and often utilized instances of fractional distillation in industries. Due to the fact that crude oil is a mixture of several materials or components, such as diesel, paraffin wax, naphtha, gasoline, kerosene, and lubricating oil. The fractional distillation method is employed in industries to separate the aforementioned components. Many steps are taken to do this. The machine’s chamber is filled with crude oil, which is heated with high-pressure steam. Once the mixture begins to boil, its vapour is produced. The vapour phase now contains a variety of substances or components and the vapour increases.
Summary
In the fractional distillation process, 2 or even more liquids with various boiling points that are close to one another in a combination may easily be separated. Vaporization is the method used for this separation. In this case, the liquid with a lower boiling point vaporizes first and separates from the mixture. The process then proceeds in a similar manner. With a pressure of 1 atm, the liquids typically have boiling points around 25 °C apart from one another. Nevertheless, simple distillation is utilized when the difference in temperature between the liquids is more than 25 °C. Separation benefits greatly from fractional distillation. Hence, it is frequently used in enterprises and refineries for oil and chemical facilities.
Frequently Asked Questions
1. Fractional distillation: Is it more precise?
Fractional distillation is more effective when the two liquids in combination have similar boiling points, often with a range not exceeding 40 degrees Celsius. With one apparatus, fractional distillation completes several straightforward distillations.
2. What is low-temperature fractional distillation?
At low temperatures, Low-temperature fractional distillation is used to separate a mixture into its constituent elements. This method is often used for the separation of component elements that boil at less than 25°C apart under a hoover.
3. Why does the temperature rise in fractional distillation in the upward direction?
Vapours move through the fractionating column during distillation, releasing their heat energy to the walls of the column and condensing. This causes the column to become warmer. The bottom end of the column is more frequently traversed by the vapours than the top end.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.



