Introduction
By treating an aryl hydrazone of a ketone/aldehyde with an acid or a variety of metal & dehydrated metal salt catalysts, \(N{H_3}\) may be removed, leading to the creation of an indole. This process is known as the Fischer indole synthesis. By directly putting an equal molar combination of aryl hydrazine as well as aldehyde/ketone into indolization settings, the actual separation of the aryl hydrazone is frequently avoided. Like this, the reduction of the appropriate aryldiazonium salt/ N-nitroso arylalkylamine in the vicinity of the CO compound results in the in-situ formation of the aryl hydrazine, which then immediately results in the aryl hydrazone. By treating pyruvic acid 1-methyl phenylhydrazone by HCl in 1883, Fischer & Jourdan achieved the very 1st indolization of an aryl hydrazone.
What is meant by Fischer Indole Synthesis?
It is one of the most effective and time-tested techniques for creating indoles. Fischer obtained the very 1st patent for it in 1883. In the introduction of acids, aryl hydrazine, as well as substituted ketone/aldehyde, can be used to create a variety of indoles. Under acidic circumstances, a phenylhydrazine, as well as a ketone/aldehyde, undergoes a chemical reaction to produce the heterocyclic aromatic indole.
The obvious benefit of this method is the usage of a wide variety of alcohols as opposed to their oxidized equivalents. The process may be completed in a single pot and offers moderate to good yields for the indoles while tolerating replacement on both hydrazines as well as alcohol. The parameters of idolization are frequently used without first isolating the aryl hydrazine using an equimolar combination of ketone/aldehyde. The same is true for aryl hydrazone, which may be immediately exposed to indolization settings in the vicinity of carbonyl without first isolating the aryl hydrazone. This is done by reducing the linked aryl diazonium /N-nitroso arylalkylamine salt.
The Fischer Indole Synthesis’s Main Features include:
- Since the aryl hydrazones don’t have to be separated, the indole synthesis may be done in a single pot.
- Unsymmetrical ketones generate 2 region-isomeric 2,3-disubstituted indoles exhibiting region-selectivity based on intermediate acidity, the substitution of hydrazine, as well as steric effects.
- 1,2-diketones can be used to produce mono- as well as bis-indoles, having mono-indoles often being formed in refluxing alcohols with powerful acid catalysts.
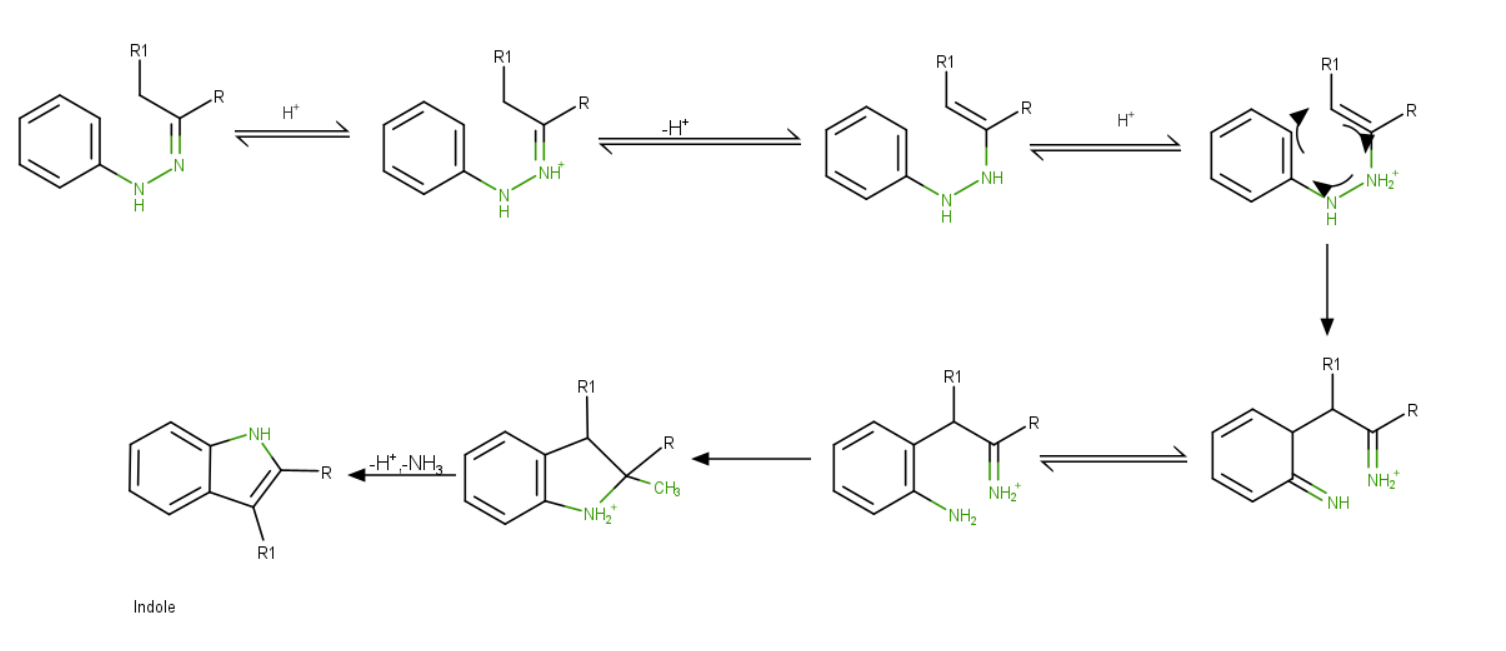
Fischer Indole Synthesis Mechanism
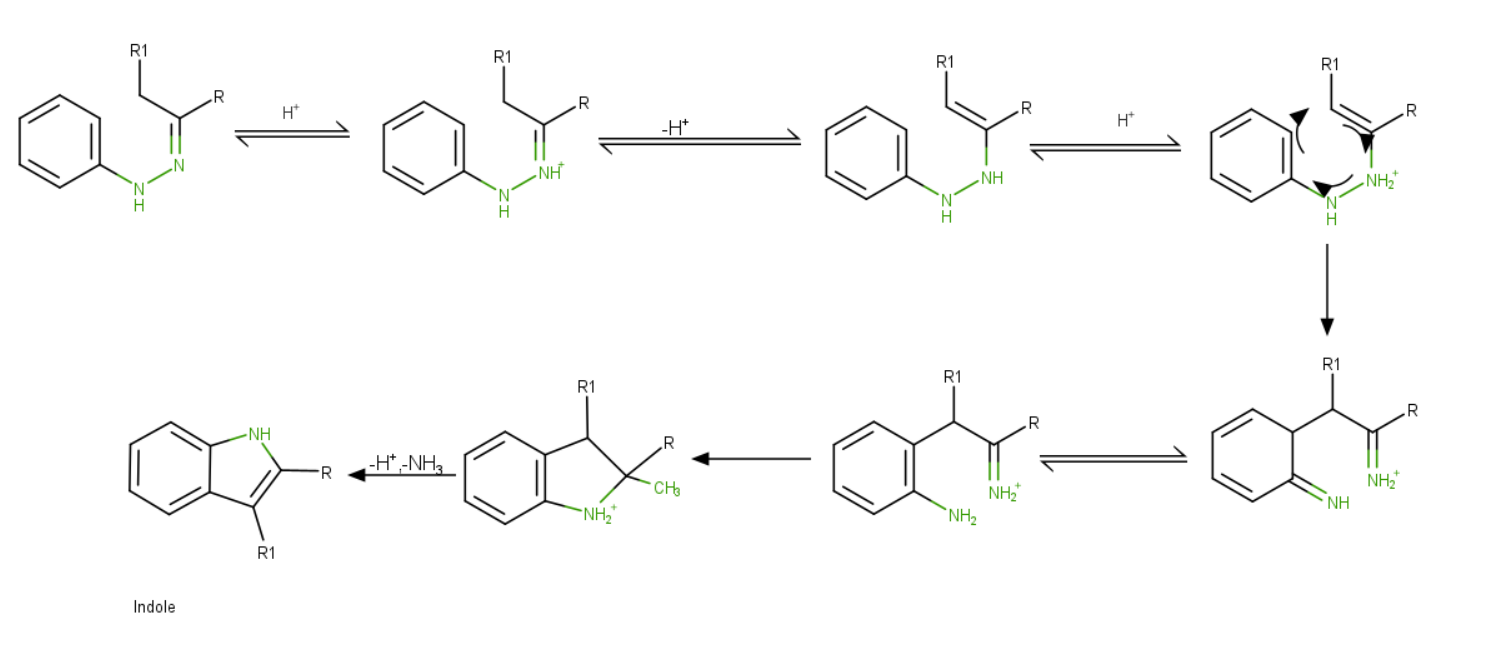
Steps:
- The product of aryl hydrazine and ketone is aryl hydrazone.
- Protonation occurs at more basic N.
- The proton is lost creating a pi bond neutralizing N to yield ene- hydrazone.
- Again, protonation at the same N and undergo [3-3] sigmatropic shift generating a C-C bond at the ortho position of the benzene ring.
- Isomerization takes place making a more stable aniline form and restoring the aromaticity of the ring.
- N from aniline moiety attacks iminium ions intramolecular fashion to secure 5 membered rings.
- One proton is lost, and acid catalyzes and by elimination of \(N{H_3}\), the final product, indole is formed.
Fischer Indolization
Fischer Indole Reaction is a convergent method that has been established to have an indole ring system that exists in many bioactive compounds. The method uses an irregular Fischer indolization sequence to condense hydrazines containing latent aldehydes. This eventually produces goods that include Indoline. The approach is convergent, gentle, simple to apply, all-encompassing, and may be applied to obtain enantioenriched products.
There has been the 1st catalytic Fischer asymmetric Indolization. When a spirocyclic chiral \({H_3}P{O_4}\) is present at a loading of 5 mol percent, it is highly enantioselective. Effective catalyst turnover has been accomplished by including a mildly acidic cation exchange resin that eliminates the ammonia produced. Different genera of three -substituted tetrahydro carbazoles are produced by the reaction, which may be carried out under favourable circumstances.
The discontinuous Fischer indolization is a powerful method for generating fused indole ring structures, which are present in several natural alkaloids. A brief description of the whole synthesis of communes is provided in alkaloids as well as perophoramidine. The method is focused on the usage of the disrupted Fischer indolization to produce the tetracyclic indole in natural products.
Fischer Indole Synthesis Procedure
Aryl hydrazine reacts with a ketone/aldehyde to produce an aryl hydrazone that isomerizes to the appropriate enzyme. A cyclic [3,3]-sigmatropic rearrangement results in the production of an imine following protonation. Under acid catalysis, the resultant imine forms a cyclic amino acetal, which removes \(N{H_3}\) to produce indole. Indole is a heterocyclic compound that is very important to biological systems. The redox-active indole side chain of tryptophan is one of the primary charge carriers engaged in the transfer of electrons in proteins. Tryptophan is one of the most important intrinsic fluorophores in the investigation of protein fluorescence due to the indole’s optical characteristics. Reductive deamination of tryptophan results in the intermediary molecule indole pyruvic acid, which is then converted into indole. The end products of the process are pyruvate, indole, ammonium, as well as water.
Summary
It can be concluded that under acidic circumstances, a phenylhydrazine, as well as a ketone/aldehyde, undergoes a reaction known as Fischer Indole Synthesis that results in the heterocyclic aromatic indole. Emil Fischer discovered the reaction in 1883. Today, this process is frequently used to create triptan-class antimigraine medications. Aryl hydrazine reacts with a ketone/aldehyde to produce an aryl hydrazone that isomerizes to the appropriate enzyme. A cyclic [3,3]-sigmatropic rearrangement results in the production of an imine following protonation. Under acid catalysis, the resultant imine forms a cyclic amino acetal, which removes \(N{H_3}\) to produce the energetically advantageous aromatic compound indole.
Frequently Asked Questions
1. Is indole basic or acidic?
Ans. Indole is a weak base, just like pyrrole. It interacts with KOH and Grignard reagents as well as polymerization by strong acids.
2. What is the Indole test?
Ans. Indole Test: This test shows that certain bacteria can break down the medium-accumulating amino acid tryptophan into indole. The test for indole synthesis is crucial for identifying Enterobacteriaceae.
3. What causes the electrophilic substitution reaction in indole?
Ans. The 3rd carbon in the indole ring is the most reactive site. This is even more reactive than benzene in terms of the electrophilic substitution process.
4. The indole ring is present in which amino acid?
Ans. L-tryptophan is the only protein amino acid (AA) with an indole ring, and when it undergoes biotransformation in living things, it either helps to preserve this chemical group in cells as well as tissues or breaks it down by producing a range of bioactive chemicals in both circumstances.
5. What reagent is utilized in the indole test?
Ans. Directly into the tube, add 5 drops of the Kovács reagent to check for the formation of indole. In a short time of introducing the reagent to the media, the reagent layer on top of the medium develops a pink to cherry-red ring, which signifies a positive indole test.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


