Introduction
An atom’s energy changes due to electron affinity. A neutral atom gains energy and a negative charge when electrons are added to its outer shell. To stabilise its octet, an element gains electrons. When an element accepts or loses an electron, energy is released. When an element accepts an electron to form a compound, it releases energy, which is referred to as an exothermic reaction. The energy is released in an exothermic reaction in order to attract the electron by a nucleus from another element. When an element loses an electron, it absorbs energy, a process known as endothermic. An atom gains energy when it loses electrons.
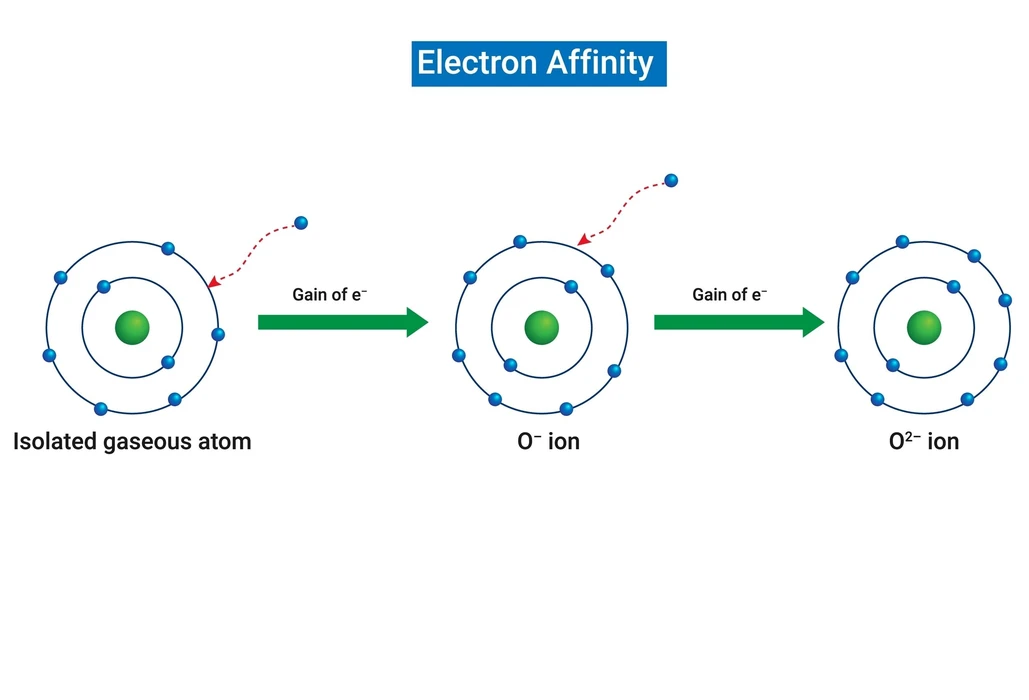
What do you mean by Electron Affinity?
When atoms accept electrons, they emit energy, which is referred to as an exothermic reaction. Atoms that lose an electron in a chemical reaction, on the other hand, absorb energy and are known as endothermic reactions. The ability to accept an electron is referred to as electron affinity. When a neutral gaseous atom accepts an electron, it gains a negative ion charge. The first electron affinity is always negative, while the second is always positive. It is difficult to measure the electron affinity of an atom. It is determined by the energy released by ionic compounds. The electron affinity is also measured by an atom’s tendency to act as an oxidising or reducing agent. It is measured in kilojoules/moles. Electron affinity is symbolised by EA.
Want to get an “A” on your Science exams? Let our expert teachers be your guide toward improving your grades and reaching your highest potential. Study Science tuition for classes 6th, 7th, and 8th.
Factors Influencing Electron Affinity
The atomic size of the element, the nuclear charge on the molecules, and the electronic configuration of atoms are all factors that influence a molecule’s electron affinity.
- Atomic size: Atoms with smaller sizes have greater electron affinity than atoms with larger sizes. The nucleus of smaller atoms is more attractive to electrons than the nucleus of larger atoms. As the atom’s size increases, the outer shell becomes further away from the nucleus, and the attraction for electrons in the outer shell decreases.
- Nuclear Charge: The nuclear charge influences electron affinity as well. As the charge on an atom increases, so does the attraction in electrons, and thus the electron affinity. When a molecule is already charged, electron repulsion increases, and the pull from the nucleus increases, resulting in increased electron affinity in charged ions.
- Shielding Effect: As the screening effect on an atom’s inner shell is reduced, the electron affinity increases.
- Electronic Configuration: The electronic configuration also affects electron affinity. Because elements with full octets have zero tendencies to accept electrons, electron affinity in inert gases is zero. The electronic configuration is crucial in electron affinity. Metals have a lower affinity for electrons than non-metals due to their electronic configuration.
Summary
The ability to accept electrons in gaseous form and form an anion is referred to as electron affinity. The process of accepting electrons generates energy, which is why it is referred to as an exothermic process. When we move from group to group, the electron affinity decreases and increases when we move from period to period. It is denoted by the symbol EA and measured in Kilojoules per Mole (KJ/Mol). Because of electron-electron repulsion, the first electron affinity is always less than the second electron affinity. The atomic size, electronic configuration, screening effect, and nuclear charge of elements all influence electron affinity.
Frequently Asked Questions
1. Why do noble gases have no electron affinity?
Ans. Noble gases have zero electron affinity because their octet is complete, and they do not have an affinity for electrons. As a result, noble gases have no electron affinity.
2. Why does group 17 have such a strong electron affinity?
Ans. Because the halogens are small and have more electrons in the outer shell, the elements of the halogens group have a high electron affinity. A halogen would rather accept an electron than lose seven electrons to complete its octet.
3. Why does fluorine have a lower electron affinity than chlorine?
Ans. Because the atomic size of fluorine molecules is smaller than that of chlorine molecules, the outer shell of fluorine is already filled with electrons, and the nucleus is much closer to the outer shell, the electron repulsion is greater than the force of attraction of the nucleus when an electron is placed in the outer shell of fluorine molecules compared to chlorine molecules.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


