Introduction
As an electromeric action, the intramolecular electron transfer phenomenon makes molecules temporarily become polarisable. This result is frequently referred to as the conjugative mechanism or the tautomeric mechanism.
It is frequently considered with other effects like an inductive effect and a mesomeric impact. Although others also acknowledge this, it is not clearly stated that the existence of the agency in question affected this. The phrase “electromeristic effect” is not even addressed in conventional textbooks. It also affects a certain form of resonance. A curving arrow is used to symbolise the electron shift that results from this occurrence metaphorically.
What is the Electromeric Effect?
Electromeric refers to the transient occurrence that includes the full transfer of electron pairs in one of the bound atoms in a multiple bonded species. They must be related to double bonds or triple bonds if they have many bonds. And it only takes place when a reagent is present. It is a transient Polaris power that was created on several coupled species, and it vanishes as the attacking reagent is withdrawn. As a result, once the attacking chemical was removed, the molecule once more reverted to its natural form. The electromeric effect has no clear direction, although an electron pair is more frequently transferred to the element with higher electronegativity. When numerous bound species exhibit an electromeric effect, their teri activity rises, and as a result, a number of new products are produced. Only organic substances contain it. Moreover, when nucleophiles or electrophiles are present, a reversible reaction takes place. The example below demonstrates the electromeric effect.
The direction of the Shift
The molecules, or the atoms linked to the bonds, determine the direction of the electron shift.
- If more of the atoms connected to the double bond share the same traits, an electron pair may flow in any direction.
- Hence, in such circumstances, the flow of electron pairs will become unpredictable.
- When the atoms bound in the numerous bonds are distinct, the movement of electron pairs becomes equivalent to the direction of the inductive effect.
- Under these circumstances, the dipole moment affects the electron flow.
Mechanism of the Electromeric Reaction
The electromeric effect’s mechanism is simple to comprehend. In that situation, the double or triple bond’s electron pair location transfers to one of the atoms that are connected to the double or triple bond. And it only takes place when an electrophile or a nucleophile is present. For instance, when cyanide is present in the process below, electron pairs are moving in favour of the atom of oxygen. And after the reagent is removed, this effect is undone.
Types of Electromeric Effect
Positive electromeric effect, abbreviated +E, and negative electromeric effect, abbreviated -E, are the two main forms of electromeric effect.
- The +E effect is seen in reactions where the reagent is an electrophile; because electrophiles are positively charged chemical species, the electron pairs in the numerous bonds are moved towards the reagent. This indicates that the provided molecule’s pair of electrons is being taken by the reagent. And the reagent helps to create a new connection.
- The -E effect occurs in a reaction in which nucleophiles are used as the reagent. The electron in the reagent will go towards the molecule in such reactions if the reagents are abundant in electrons. Considering that negatively charged organisms seek out a bright side. An illustration of a detrimental electromeric action is the addition of cyanide to ketones and aldehydes.

Positive Electromeric Effect
Examples of the Electromeric Effect
Here are some instances of reactions that exhibit electromeric effects.
- The electromeric effect is produced by the nucleophilic addition of certain nucleophiles to the carbonyl compounds. Nucleophiles are added to the positive side of carbonyl compounds because they are negatively charged.
- Electrophiles and nucleophiles can be found in hydrogen halides. They contain an electrophile that attacks the electron pair, removes the electron, and causes the creation of a bond. The reaction is finished when a nucleophile is present.
- Polarisation is produced by the electrophilic addition process, which involves adding an electrophile to symmetrical alkynes or alkenes.
- Benzenoids undergo electrophilic substitution reactions: The presence of electrophiles causes benzene to become polarised
Differences Between Electromeric Effect and Inductive Effect
These two impacts differ in a few ways, which are shown below in a table:

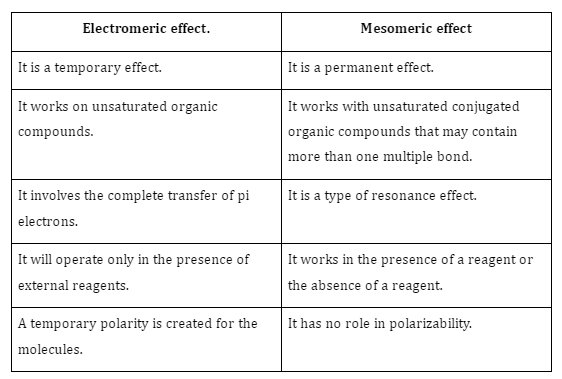
Difference Between Electromeric and Mesomeric Effects
The differences between the electromeric and mesomeric effects are given below:
Summary
A phenomenon known as the electromeric effect occurs in unsaturated chemical molecules. The development of polarisability is a transient phenomenon. They fall into one of two categories: electromeric effects, both positive and negative. These occurrences only take place when an outside reagent is present. These substances might be electrophilic or nucleophilic. Numerous instances demonstrate the electromeric effect. Examples of this phenomenon include electrophilic and nucleophilic substitution. This reaction’s mechanism is quite straightforward to comprehend. It differs greatly from other effects like the mesomeric effect and the inductive effect. Also, it has a reverse impact.
Frequently Asked Questions
1. How come the electromeric effect is transient?
After the attacking agent is eliminated, the pi electron pair returns to its original place and forms another multiple bond. The electromeric effect is thus a transient phenomenon, and the resulting product can not be isolated
2. Does the +E effect make the substrate a nucleophile?
No, the +E effect makes the substrate an electrophile. This is because there is a decrease in the electron density on the aom undergoing +E effect as a result of electron donation. The substrate can easily be attacked by a nucleophile
3. Which effect is more stabilizing electromeric effect or hyperconjugation?
The electromeric effect is more stabilizing than the hyperconjugation effect. This is because electromeric results in conjugation, which leads to the release of a lot of excess energy. Hyperconjugation involves no pi-electron conjugation.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


