Introduction
The electron (e-) is a particle of an atom, just as an atom is a substructure of matter. There is no way to create or destroy these atoms. Atoms were assumed to be the smallest unit of a particle before the discovery of electrons (e-). Tests used in the discovery of the electron shed light on some of the atomic basic features (e-). It was a major turning point in physics when the electron (e-) was discovered as the first subatomic particle. It turns out to be one of the most crucial in modern chemistry and physics for defining the chemical bond.
Thomson Cathode Ray Experiment
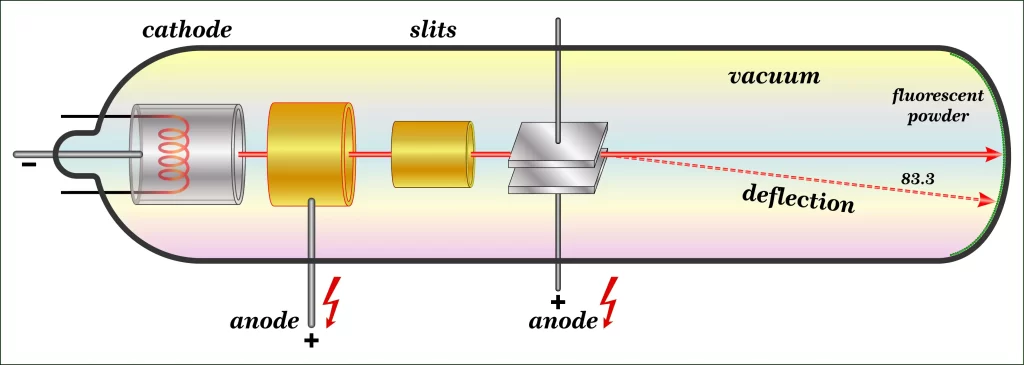
J.J. Thomson (J.J.T.), a physicist, studied CRTs (cathode ray tubes) in the early 1990s (CRT). A glass tube, sealed under vacuum, is present. Particles are accelerated from the cathode to the anode as a high voltage is supplied between two electrodes on one side of the tube.
Cathode-ray tubes get its name from the fact that the cathode is the point of genesis for the particle beam (CRT). Phosphors produce a spark or light when hit by the beam. Thomson investigated the particles’ properties by surrounding the beam with two electric plates that were charged in the opposite direction. A positive electric plate was used to deflect the beam away from a negatively charged one. This means that the particles in the beam were all electronegative.
He also found that the magnetic field could be used to redirect the beam by placing magnets on each side of the tube. After calculating the mass-to-charge ratio of beam particles, he conducted an experiment showing that the mass of a single particle was far less than that of any known atom. Cathode Ray (CR) attributes were the same and irrespective of cathode composition, he found after conducting more tests using metallic elements as electrode components. The following conclusions were then made:
- Components of the cathode ray are all of the negative variety.
- Particles must have been part of the atom, as their combined mass is 1 x 2000 that of a single hydrogen atom.
- Every atomic nuclei have the potential to contain these subatomic particles.
His findings were originally controversial, but experts gradually accepted them. This cathode ray (CR) particles were subsequently referred to as electrons (e–).
Cathode Ray Experiment
Define Electrons
The electron (e-) is a negatively charged subatomic particle with a small mass. Consequently, it may be readily deflected by approaching another e- or the positive nucleus of an atom. The discovery of this primary building block came first. These are fundamental particles with a negative one-charge. An e- has the same negative charge as a proton (but has an opposite sign).
While protons and e– have the same charge magnitudes, the former is substantially smaller and lighter than the latter. An e- is a particle with a negative charge. The negative charge is \(1.602 \times {10^{ – 19}}\) Coulomb in magnitude. The mass of an e- is just 1/1837 that of a proton.
Electron density: The mass of one electron is \(9.10938356 \times {10^{ – 31}}kg\)kilograms (e-). As compared to the mass of a proton, the mass of an electron (e–) is extremely small.
Properties of Electrons
Following are the properties of electrons:
- The positively charged protons and neutrons make up the nucleus of an atom, while the negatively charged electrons orbit around it.
- Electrons are the sole fundamental component of an atom that cannot be further split into smaller subatomic particles like protons and neutrons. The letters e or e- stand for them in the alphabet.
- It is possible to raise the energy level of an electron by absorbing some of its lower energy level.
- Due to their extremely low mass of 9.10941031 kg, electrons do not contribute to an atom’s total mass.
- A proton has a positive electric charge of +1, whereas an e- has a negative charge of -1.
- The attraction between the negative charge of the electron and the positive charge of the protons causes the electron to go in a certain orbit around the nucleus.
Electrons and Compounds
The electron was discovered in 1897. The discovery of the proton between 1911 and 1919 and the neutron in 1932, however, recast the electron’s meaning and significance. The whole concept of atomic structure was established with the discovery of the other two subatomic particles.
The nucleus of an atom is composed of positively charged subatomic particles called protons. The number of protons in an atom and the size of an electron are both element-specific. For comparison, oxygen (O) has 8 protons and carbon (C) has 6, but hydrogen (H) has just 1.
Rutherford postulated neutrons before discovering the proton; Chadwick confirmed their existence in 1932. As neutrons have no net electric charge, they were appropriately named. All elements in the periodic table are made up of neutrons except hydrogen (H). A neutron’s mass is somewhat more than that of a proton.
Summary
Electrons were first identified as particles in cathode rays, discovered by J.J. Thomson, whose discoveries were widely accepted. As a result of his research, a revised model of the atom’s structure was created. The electron’s charge was first determined by American scientist Robert Millikan. Using electrically charged oil droplets, he determined the charge on a single electron. He determined that the charge of a single e- is \(1.602 \times {10^{ – 19}}\) Coulomb. Millikan determined the electron’s mass by calculating its charge and utilising J.J. Thomson’s mass-to-charge ratio. The mass of an electron is \(9.1094 \times {10^{ – 31}}kg\) kg. In 1906, his discovery of the electron earned him the Nobel Prize in Physics.
Frequently Asked Questions
1. Why do electrons have a “negative” charge?
As electrons repel an electric field, the negative connotation is derived from this fact. By convention, a negative charge is assigned when an electron moves from the negative to the positive pole of an electric field.
2. How are electrons able to travel about?
When an electric voltage is applied, an electric field is created within the metal. This electric field causes the flow of electrons, which causes the electrons to migrate from one end of the conductor to the other end. The electrons will migrate towards the positive side of the charged object.
3. Do cathode rays have the ability to go through glass?
Cathode rays are not visible to the human eye, but when they strike the glass wall of an early hoover tube, they excite the atoms there, which in turn causes the atoms to fluoresce, another name for the emission of light.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


