Introduction
There are two types of acids, concentrated and dilute, both of which are defined by their concentration. Dilution occurs when an acid has a significant amount of water added to it. As such, they are diluted to lower the overall acidity. We stay away from the very dangerous acids. In addition, we pay special attention to acids because they may be kept in the home for regular use. Also, they are employed in the control of several chemical operations.
What are Acids?
To be considered acidic, a substance must have a sour flavour when dissolved in water, change the colour of certain indicators (like blue litmus turning red), react with certain metals (like Fe) to release H+ions, react with bases to generate salts, and speed up chemical processes. Acids can be either inorganic (such as nitric and phosphoric acids) or organic (such as phenolic and sulfonic acids) molecules. When these compounds are dissolved in water, the H atom(s) they contain are released as positively charged H+ions.
Examples of Acids
Examples of acids include the following:
- Arrhenius acid
- Citric acid
- Hydrochloric acid
- Sulfuric acid
- Acetic acid
- Vinegar
What is Dilute Acid?
If an acid has been diluted, it has lost more of its acidity to water than the water has gained from the acid. It does not weaken acid or reduce its reactivity. The acidity of the solution you’re working with will decrease. Dilute acids include sulfuric acid that is just 5 percent concentrated. The concentration of sulfuric acid in a solution of 100 grams of water is five percent.
Properties of Dilute Acids
Some common properties of acids are:
- There is a sour aftertaste while consuming acid.
- Electrical solutions are formed when water and acids are mixed together. All of the anionic charge is lost when strong mineral acids are dissolved in water. When organic acids are dissolved in water, a mild ionisation occurs. Some molecules are still in the process of joining together. They are referred to as weak acids.
- When exposed to acids, the litmus indicator changes from blue to red.
Chemical Properties of Dilute Acids
Hydrogen gas is created when metals react. Get some zinc flakes and place them in a clear test tube. Fill the test tube with a solution of HCl that has been diluted. The reaction produces a gas. A burning splitter is moved extremely close to the mouths of the test tubes in order to examine hydrogen gas. A popping noise can be used to detect the presence of hydrogen gas.
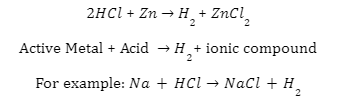
Salt & water are produced when an acid reacts with a base (metallic oxide).
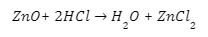
When acids react with sodium hydroxide, salt and water are produced.
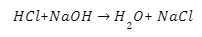
Altering the pH of the reaction environment can cause profound changes in the products generated from two extremely specific reagents.
Examples of dilute acids
Dilute Hydrochloric acid
Hydrochloric acid, often known as muriatic acid, is an aqueous solution of hydrogen chloride. The chemical symbol for dilute hydrochloric acid is HCl. The solution lacks colour and has a distinct aroma. Hydrochloric acid is a strong acid. It is a part of the stomach acid of most animals, including humans. Hydrochloric acid is a crucial reagent in many different industries and fields of study. Dilute hydrochloric acid refers to aqueous acid mixes in which the concentration of HCl is lower than that of water.
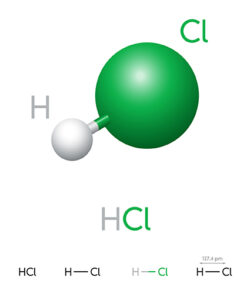
HCl molecule
Dilute sulfuric acid
Sulfuric acid is a mineral acid made up of H2O and sulphur. Sulfuric acid has the formula\({H_2}S{O_4}\). Sulfuric acid is also known by the name “oil of vitriol,” which is a synonym. It is a thick liquid that is both odourless and water-soluble. With its hygroscopic properties, it swiftly soaks up moisture from the air. The aqueous form of sulfuric acid is a powerful acid.
Dilute acetic acid
Ethanoic acid is the official word for acetic acid. \(C{H_3}COOH\) is the molecular formula. It’s an acidic, odourless, and colourless organic molecule. Vinegar contains just acetic acid and water, with the acetic acid accounting for at least 4% of the liquid content. Acetic acid ranks as the second simplest carboxylic acid. The hydrogen in the centre of the carboxylic group is ionisable by carboxylic acids like acetic acid (COOH). Due of this hydronium ion release, acetic acid is acidic. Disappointing monoprotic acid quality.
Summary
Hence, acids are compounds that, upon contact with a base, give forth hydronium ions. Protons are donated to the body by acids. pH values below 7 are considered acidic. Substantial volumes of water are found in diluted acids. A concentrated acid can be diluted using water. A lot of hydrogen ions are released into the water when a strong acid is used. Inorganic acids such as sulfuric, nitric, and hydrochloric acid are examples of strong acids. These substances are called weak acids because they only dissociate into a small number of hydrogen ions when dissolved in water.
Frequently Asked Questions
1. What is the procedure of dilution?
Ans. Acid is always poured into the water and not the other way around during dilution. When \({H_2}O\) is combined with an acidic solution, the \({H^+}\) ion concentration decreases, and the solution’s pH rises. The acidic strength then starts to weaken and it becomes diluted.
2. Are diluted acids as hazardous as concentrated acids?
Ans. Although dilute acids are considerably less hazardous than concentrated counterparts, they are labelled with a warning that they could cause mild health problems or skin irritation. This makes sure that if one of them touches your skin, it will get red or blister.
3. How are dilute acids conducting in nature?
Ans. Dilute acids have free ions in the solvent. These ions carry charge from one electrode to another on application of potential and conduct electricity. Aqueous HCl solutions can generate energy. Diluted acid produces fewer ions and conducts electricity less.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


