Introduction
Evaporation is the transformation of liquid into a gaseous state. The simple process of water evaporating from the soil in response to the sun’s heating influence can help us grasp the concept of evaporation. By contrast, “boiling” refers to bringing a liquid to a temperature above its boiling point. There is a significant difference between evaporation and boiling, with the former affecting only the liquid’s surface and the latter affecting the bulk of the substance.
In most people’s minds, this is the main difference between boiling and evaporation. In contrast to boiling, evaporation occurs naturally and unintentionally. Therefore, we need to distinguish between the two terms to get a proper grasp on them.
The solid-state
Densely packed particles create a solid state. In the solid state, the positions of the particles within a substance are fixed concerning one another due to the lack of thermal energy to break the intermolecular connections between them. It follows that solids are distinguished by their shape and volume. The particles that make up a solid form a crystal when they arrange themselves in a repeating, three-dimensional pattern of positive and negative ions.
The liquid state
If the particles in a substance have enough energy to partially overcome intermolecular interactions, they can move around each other while still being in contact. This is the state of matter known as a liquid phase. Given that the particles in a liquid are still relatively close together, the volume of the liquid is fixed. Since the particles in a liquid can move relatively freely around each other, the liquid can take on the shape of its container.
The gaseous state
The gaseous phases lack a distinct structure and volume. It occupies the entire space of the container.
What is boiling?
Constant heating causes a liquid to boil and transform into a gas. When the temperature hits boiling, it happens, and bubbles are produced. At this point, a liquid boils and rapidly loses its liquid state.
If you heat a liquid to its boiling point, its particles will start moving more rapidly and agitating. Remember that, unlike evaporation, boiling is usually not a natural occurrence. When the external pressure on a liquid equals the vapour pressure of the gas it’s giving off, we say that the liquid is boiling.
Boiling
What is the boiling point?
The boiling point is the temperature at which the liquid starts boiling. The temperature doesn’t change once the liquid begins to boil until all of the liquid has been transformed into a gas.
What is evaporation?
When a liquid changes into a gas because of high pressure or temperature, this natural process is called evaporation. It does not matter what the temperature is. Even more so, bubbles are not produced during the evaporation process. Evaporation, a natural phenomenon, plays a key role in the water cycle. It can happen at any time, regardless of how hot it becomes. Leave a glass of water out on the table for a while and you’ll see that the water level gradually decreases without any help from you. It’s one of the two ways that things can be vaporised. Atoms or molecules in a liquid state are given enough energy to undergo a phase transition into the gaseous state.
Factors affecting evaporation
- Temperature: The rate of evaporation depends on the ambient temperature. A liquid changes into a gas in the presence of increasing kinetic energy. Therefore, evaporation occurs at a faster rate.
- Surface area: How quickly a liquid evaporates is directly proportional to the amount of surface area it is exposed to. A common method for expediting water evaporation from a wet cloth is to stretch it out past the cloth line.
- The humidity of air: Evaporation is significantly affected by the humidity of the surrounding air. The more time it takes for our clothes to dry after being wet, the more water vapour will be in the air.
- Wind speed: The rate of evaporation increases with the strength of the wind. Water evaporates more quickly when there is more wind, which increases the kinetic energy between the water molecules.
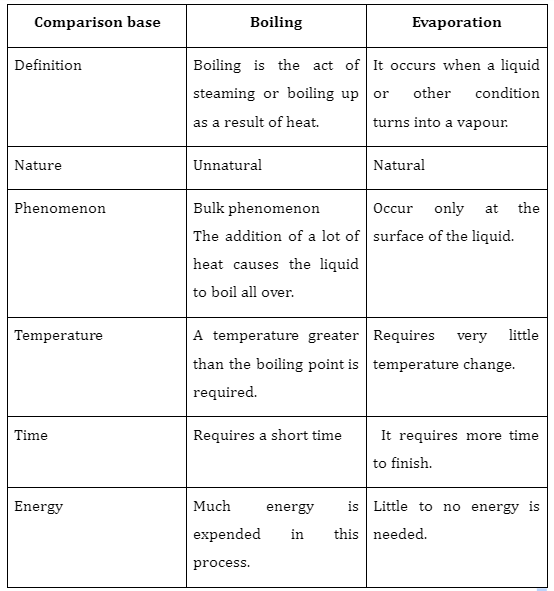
Difference between evaporation and boiling
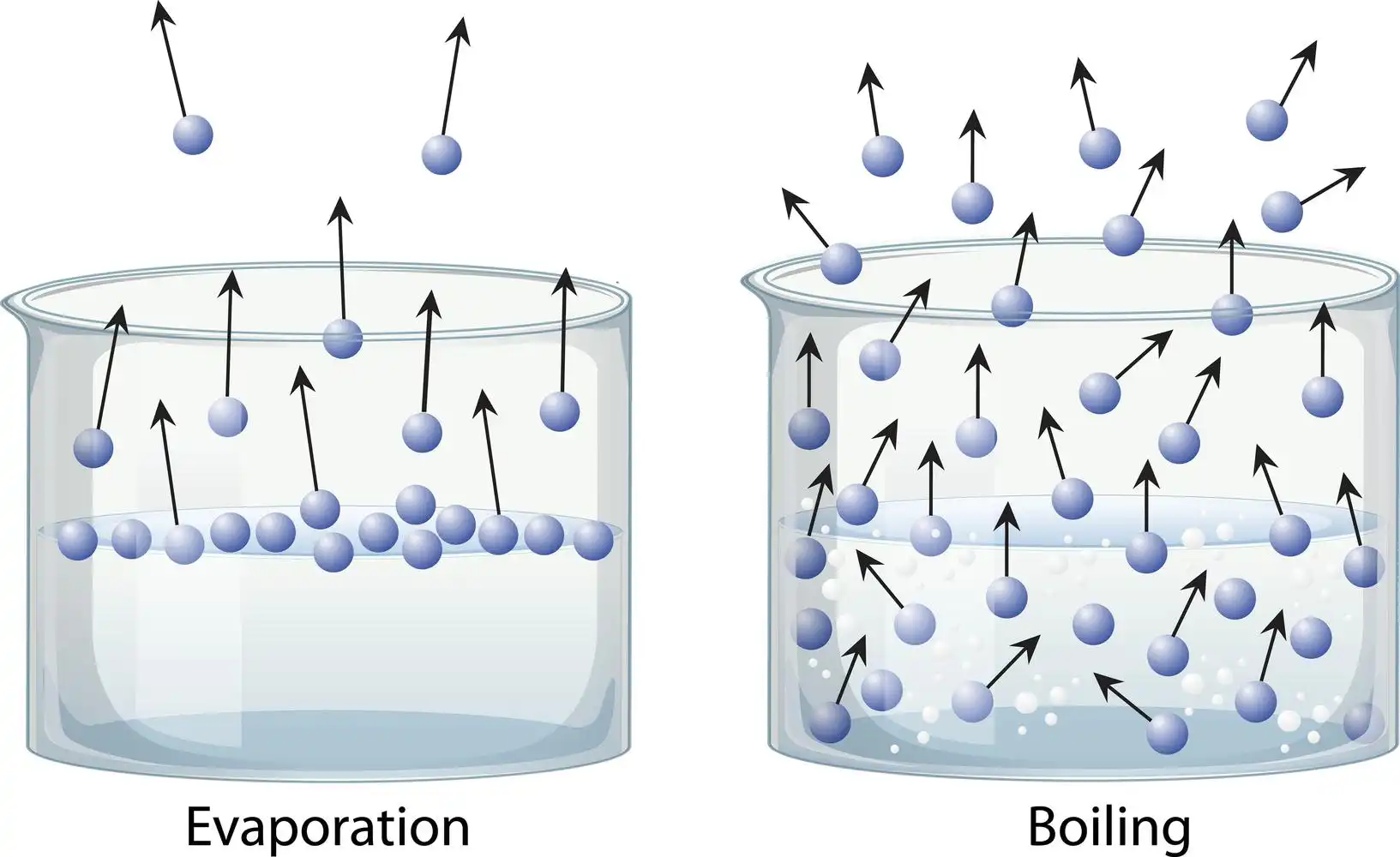
Summary
In conclusion, evaporation is slower, only occurs from the liquid’s surface, doesn’t result in bubbles, and causes cooling. Boiling is quicker, can happen anywhere in the liquid, results in a lot of bubbles, and does not cause cooling. Evaporation is a typical process that takes place when a liquid transforms into gas while raising the temperature or pressure. Boiling is an unnatural process in which the liquid is continuously heated to a point where it evaporates. The temperature at which a liquid’s vapour pressure equals the surrounding pressure is known as the boiling point. The boiling point falls off as the altitude rises.
Frequently Asked Questions
1. Give some illustrations of evaporation.
Evaporation can occur when ice cubes begin to melt, for instance. Another example is the drying of damp surfaces such as floors and clothing. Another example is the evaporation of nail paint remover. Others include iced drinks, clothing ironing, drying damp hair, and more.
2. What distinguishes evaporation from vaporisation?
Molecules may also emerge from below a liquid’s surface during vaporisation. Only the molecules on the liquid’s surface evaporate when a liquid is evaporating. Both vaporisation and evaporation are simply phases of a substance changing from a solid or liquid state to a gaseous state.
3. Which two vaporisation types are there?
Evaporation and boiling are the two different types of vaporisation that exist. Only during the phase transition between the liquid and the gaseous phase can evaporation or a surface event takes place. The molecules or atoms on the surface gain energy from their surroundings, defeat the pull of other molecules and become vaporised.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


