Introduction
An atom is the smallest unit of matter that preserves all the features of its element, while a molecule is a compound made up of numerous bonded atoms. Atoms and molecules are related, notwithstanding their differences. Atoms are the smallest unit of matter, while molecules are made up of several atoms, therefore they are clearly different from one another. A tomos, from the Greek a-tomos, means “indivisible,” which is apt because atoms are indivisible. Therefore, molecules can be further divided but atoms cannot.
Definition of Atom
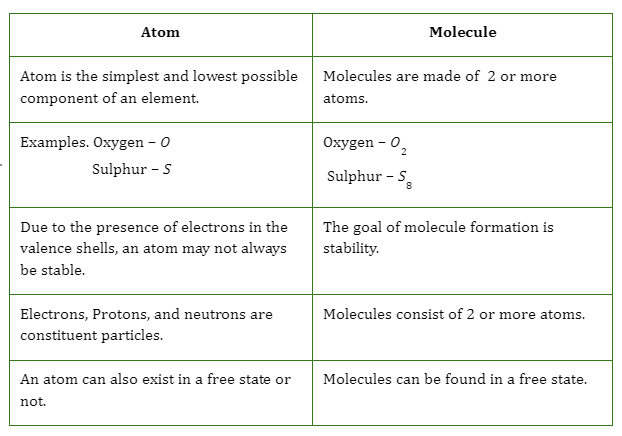
Elements can be identified by their unique atoms, which are stable and resistant to chemical breakdown. Atoms typically consist of a nucleus composed of neutral protons and neutrons, with electrons carrying negative charges and circling the nucleus. The size of an atom is dependent on its number of protons and neutrons, as well as the existence or absence of electrons. The typical size of an atom is around 100 picometers, or 1/10 billionth of a metre. Nucleus mass is virtually entirely due to protons and neutrons because electrons contribute so little.
Atomic Structure
Features of atom on the bases of modern atomic theory
- The term “modern atomic theory” is used to describe the most up-to-date, canonical explanation of atoms.
- According to the foundations of atomic theory, atoms are the smallest units of chemical matter. They are the most basic building blocks of chemistry; they cannot be broken down any more.
- Each element has its own distinct atomic structure, which differs from that of every other element.
- Although, atoms can break down into much smaller particles. The nucleus of every element contains the same amount of protons, which are positively charged subatomic particles.
- Neutrons are also present in the nucleus, albeit the exact number varies amongst isotopes of the same atomic type.
- There are two types of atoms in the universe: isotopes, which have a varied number of neutrons but the same number of protons. For example, whereas all hydrogen atoms share a single proton, hydrogen-2 also has a neutron while hydrogen-1 does not.
Proton, electron and neutron
Introduction of Molecule
Atoms of a molecule are held together in a certain configuration by chemical bonds. A molecule, like\({O_2}\), can consist of two or more atoms of the same element or of atoms from different elements. The properties of a chemical depend on the arrangements of its atoms within its molecule. The molecular weight is comparable to the sum of the atomic weights of the molecule’s constituent elements.
Bonding in Atoms
A molecule will be formed by bonding of two or more atoms. The atomic bonding is of several types such as:
Ionic bond
By sharing electrons between atoms, an ionic bond is formed. An ionically bonded substance is salt (NaCl), for instance. One of sodium’s outermost electrons is given up to chlorine so that the latter can finish filling its shell.
Covalent bond
To form a covalent bond, two or more atoms must share electrons from their outermost shell. Polymers are an example of materials that use covalent bonding. Polymers typically consist of long chains of hydrogen and carbon atoms connected via covalent bonds.
Metallic bond
When the electrons in the outermost shell are not paired with any particular atom or ion but instead exist as a “cloud” of electrons surrounding the ion centres, a metallic bond is formed. Magnesium, sodium, and aluminium are all examples of elements that form metallic bonds.
Difference between Atoms and Molecules
Summary
According to scientific consensus, the smallest unit of an element that can or cannot exist freely is an atom. Instead, the smallest unit of a compound is a molecule, which consists of a collection of atoms bound together by chemical forces. It’s possible for an atom to be either free or bound. To be sure, molecules exist in a liberated form as well. Aside from that, an atom has a nucleus filled with protons and neutrons and electrons around it. Conversely, molecules are made up of two or more atoms that are chemically bound together and may share some or all of their properties.
Frequently Asked Questions
1. Are there no forces of attraction between the molecules of inert gases?
Ans. Inert gases have stable molecules that are not attracted to one another by electrostatic forces but do have weak van der Waals attractions and London dispersion forces.
2. Is polar bond an ionic bond ?
Ans. No, a polar bond is not an ionic bond. It is a specific kind of covalent bond which is formed between an electronegative and an electropositive atom .
3. What is the difference between positron and electron?
Ans. Electrons having the opposite chirality or quantum spin are called Positrons. The polarity of the electric charge is reversed since the spin is anticlockwise. Electrons are found inside an atom while positrons are not.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.