Introduction
Organic compounds are those in which one or more than one carbon atom is connected with other elements like hydrogen, oxygen, or nitrogen via covalent bonds. These organic molecules can be divided based on their structure and the presence of different functional groups. Methane is the smallest organic compound.
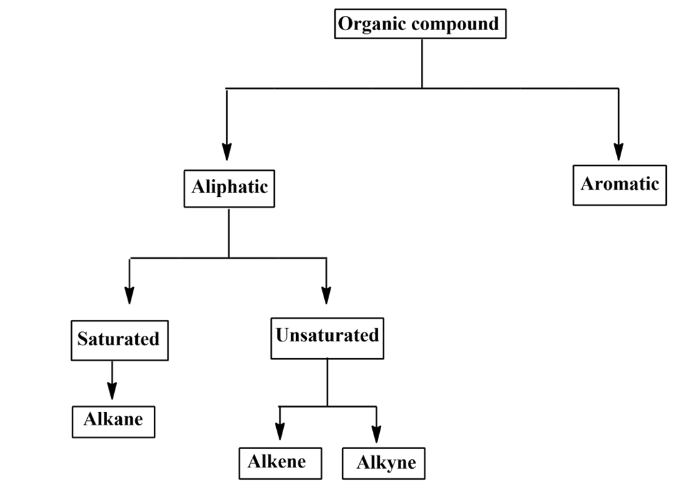
Classification of organic compounds
Organic compounds, mainly hydrocarbons can be divided into two parts:
- Aliphatic hydrocarbons
- Aromatic hydrocarbons.
- Aliphatic hydrocarbons: These are the compounds, drawn in an open-chain format. They never form any ring as they form linear structures. They can be classified into two types:
- Saturated compounds.
- Unsaturated compounds.
- Saturated compounds: Saturated compounds are those in which two carbon atoms are linked together only via a single bond. Alkane falls under this category.
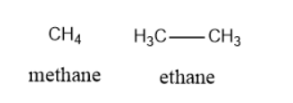
- Alkane: In this type of structure, C atoms form single bonds with the H atoms. For example Methane, Ethane, Propane, Butane, etc.
\[\begin{array}{l}Methane:C{H_4}\\Ethane:{\rm{ }}{C_2}{H_6}\\Propane:{\rm{ }}{C_3}{H_8}\\Butane:{\rm{ }}{C_4}{H_{10}}\end{array}\]
Physical characteristics of Alkane:
- Alkanes are colorless and the density of alkanes is very low.
- The alkanes, containing a lower number of carbon atoms, have lesser boiling and melting point. But with an increase in the number of carbon atoms in hydrocarbons, melting and boiling point increases.
- Alkanes are non polar. So they always tend to be dissolved in nonpolar solvents.
Chemical properties of Alkane:
- The reactivity of alkanes is very low.
- Alkane doesn’t react with any kind of strong acids or bases. It is also unreactive toward any reducing or oxidizing agent.
- Alkanes are combustible and produce heat.
- Alkanes can undergo reactions with halogens only in presence of UV light.
Unsaturated compounds: In unsaturated compounds, two adjacent carbon atoms are joined together by a double bond or a triple bond. The addition of hydrogens to unsaturated hydrocarbons leads to the formation of saturated hydrocarbons. They are classified into two groups:
- Alkene
- Alkyne
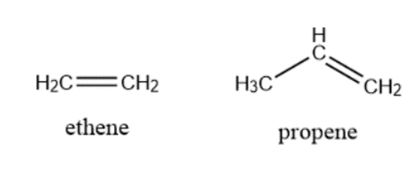
Alkene: Compounds that hold one or more double bonds between two carbon atoms are called alkenes. For Example Ethylene, Propyne, etc.
\[\begin{array}{*{20}{l}}{Ethylene:{\rm{ }}{C_2}{H_4}}\\{Propene:{\rm{ }}{C_3}{H_6}\;\;}\end{array}\]
Physical characteristics of alkene
- The first three alkene group members are gaseous, the following fourteen are liquids, and the final three alkenes are solids.
- Like alkanes, alkenes are nonpolar and dissolve in highly non-polar solvents like benzene,
- With an increase in the number of carbon atoms in alkenes, the boiling point increases. Similar to alkanes, straight-chain alkenes have a higher point of boiling than branched-chain alkenes.
- The arrangement of the molecules impacts the melting points of alkenes. However, the melting point of the trans isomer is higher than cis.
Chemical features of alkenes
- Alkenes undergo addition reactions with hydrogen molecules, halogens, and water molecules.
- Alkenes are more reactive than alkanes. Alkenes burn at a much faster rate with oxygen.
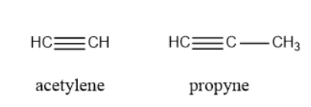
Alkyne: The compounds that contain one or more than one triple bond are called alkyne systems. For example Acetylene, Propyne, etc.
Physical properties of alkyne
- Alkynes are unsaturated, nonpolar hydrocarbons with characteristics that are identical to those of alkanes and ketones.
- Alkynes are insoluble in water, weakly soluble in polar solvents, and soluble in organic solvents.
- Alkynes have a somewhat larger boiling point than alkanes and alkenes.
- Due to the linear structures of alkynes, the molecules are very closely arranged. So their melting point and boiling point are higher than alkane and alkyne.
Chemical properties of alkynes
- It can undergo addition reactions with electrophilic substances like HCl or HBr and Br2, I2, etc.
- The additional pi bonds in alkynes, then alkenes, make them the most reactive. towards chemical reactions.
- Due to the linear structures of alkynes, the molecules are very closely arranged.
Aromatic hydrocarbons: These hydrocarbons contain sigma bonds along with the delocalization of pi electrons in a cyclic rearrangement. It is another kind of unsaturated compound. For example Benzene.
Characteristics of Aromatic compounds:
- These are cyclic and planar in structure.
- They consist of only carbon and hydrogen atoms. The double bonds are conjugated in these structures.
- These compounds possess a unique odor or aroma. So they are called aromatic.
- They are nonpolar and due to their un reactivity, they are used as a solvent for dissolving non-polar compounds.
- They are more volatile than aliphatic compounds.
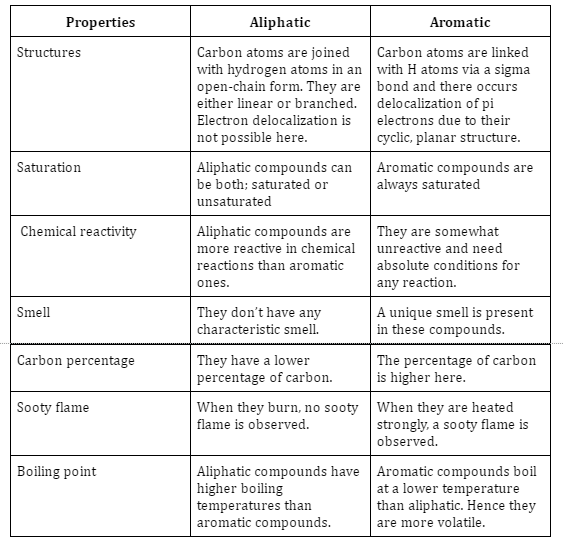
Difference between aliphatic and aromatic hydrocarbons
Summary
Organic compounds mainly hydrocarbons can be classified into two groups; Aliphatic and Aromatic. Aliphatic compounds are of two types; saturated and unsaturated. Alanes fall under the category of saturated hydrocarbons whereas alkenes and alkynes are part of unsaturated hydrocarbons. There is a large difference between aliphatic and aromatic compounds based on their chemical and physical characteristics. Classification of organic compounds is really important. As different types of organic compounds have different chemical properties, hence they can participate in the reaction with the same chemicals but follow different mechanistic steps. So to determine the mechanism of any reaction, we need to know about different kinds of organic molecules. That is why the classification of organic compounds is one of the most important topics for chemists.
Frequently Asked Questions
1. What is the drawback of the classification of organic compounds?
Ans: Carbonate and cyanide salts are also carbon-containing compounds. But the compounds like hydrogen cyanide and carbon dioxide are not considered to be organic molecules. This is the drawback of the classification of organic compounds.
2. What are the necessary features of an organic compound?
Ans: The compounds in which carbon atoms are covalently linked with hydrogen, nitrogen, and oxygen atoms are called organic compounds. In hydrocarbons, carbon atoms are linked with hydrogen atoms only.
3. Which organic molecule provides energy?
Ans: Carbohydrate compounds can provide energy. For example, a monosaccharide like Glucose can provide energy very fast as it can be digested quickly.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


