Introduction
Aufbau is a German word that means “building up.” Like a construction build-up from the ground up. Atoms are also filled with electrons in this manner. An atom has orbitals that are arranged in increasing energy level order. According to the Aufbau principle, electrons are filled in the order of increasing energy of the atomic orbitals. That is from the bottom to the top. This principle aids in the electronic configuration of atoms as well as the placement of electrons in orbitals. In all atoms, the orbital is always the first orbital to be filled with electrons. After filling this orbital, electrons are filled in orbitals further away.
Explain Aufbau Principle
Niels Bohr, a Danish physicist developed the principle. According to this principle, the increasing order of energy levels of atoms causes the filling of electrons in an atom. They are entering a perfect order that corresponds to the energy level of orbitals. We can predict the electron configurations of atoms or ions by using this rule.
The Madelung rule or rule is also related to this rule. According to this rule, the filling of electrons in an atom occurs as the value of n+l increases. That is, the electrons are filled to a lower-valued orbital. Where n represents the principal quantum number value, and l represents the angular momentum quantum number value. This is known as the Madelung rule or the diagonal rule.
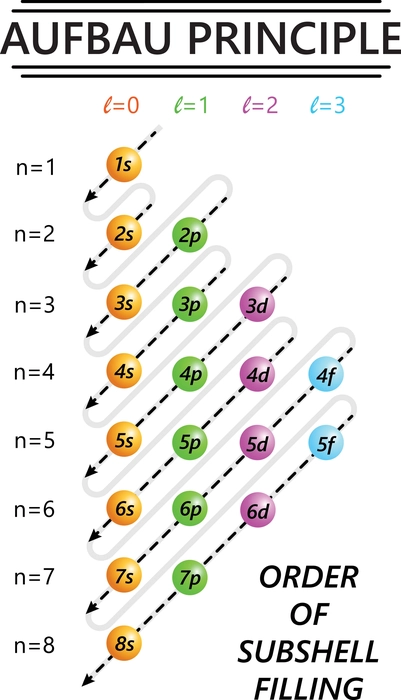
Some features of the Aufbau Principle
- Electrons are assigned to the subshell with the lowest energetically available energy.
- An orbital can only hold two electrons.
- If two or more energetically equivalent orbitals (e.g., p, d, etc.) are available, electrons should be spread out before being paired up (Hund’s rule).
Some Exceptions
Some elements exhibit exceptional behaviour in terms of the Aufbau principle. They are chromium and copper, respectively. According to the Aufbau principle, the electronic configuration of chromium is \(\left[ {Ar} \right]3{d^4}4{s^2}\). However, chromium’s electronic configuration is \(\left[ {Ar} \right]3{d^5}4{s^1}\). And this is because chromium achieves stability by having a half-filled orbital. Elements require a filled state at all times. A fully-filled orbital is always more stable. Even though a half-filled orbital has partial stability.
Copper’s electronic configuration is \(\left[ {Ar} \right]{\rm{ }}3{d^{10}}4{s^1}\) rather than \(\left[ {Ar} \right]{\rm{ }}3{d^9}4{s^2}\). This is due to the presence of a fully-filled d-orbital configuration, which provides additional stability.
Summary
An electronic configuration is present for all elements to locate electrons in orbitals. As a result, the chemical properties of elements can be explained. When combined with other rules, this can result in a proper electronic configuration. According to Aufbau’s principle, the filling of electrons in an atomic orbital occurs in the order of increasing energy of atomic orbitals. The elements chromium and copper are exceptions to this rule. Because they achieve a half-filled and fully-filled atomic orbital, these elements can be more stable.
Frequently Asked Questions
1. Define Hund’s rule of maximum multiplicity
For an orbital of the same sub-shell, the filling of electrons takes place in a way that all the electrons are singly occupied before pairing occurs. The pairing of electrons takes place only when all the subshells are singly occupied.
2. What do you understand by Pauli’s exclusion principle?
All the quantum number values are distinct for each electron present in an atom. This principle states that no two electrons in an atom can have an equal set of all the quantum number values. And thereby we can easily locate all the electrons in an atom.
3. What is the principal quantum number?
The number that deals with the energy and size of orbitals are a principal quantum number. It will explain how far an electron is from the nucleus. For example, the electronic configuration of Helium is \(1{s^2}\) so the principal quantum number is 1.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.