Introduction
It is possible for elements in nature to change into another by various processes. Such processes are classified as nuclear reactions. For instance, nuclear decay is a type of reaction which occurs when an unstable nucleus emits energy, nucleons, and/or atoms to achieve stability. Various other types of reactions also exist.
Various types of nuclear decay can occur in nature and one of those is alpha decay. This process occurs when a nucleus emits an alpha particle to gain stability. It might be worth mentioning that an alpha particle is very much like a Helium atom with a charge of +2e, i.e., two protons and two neutrons.
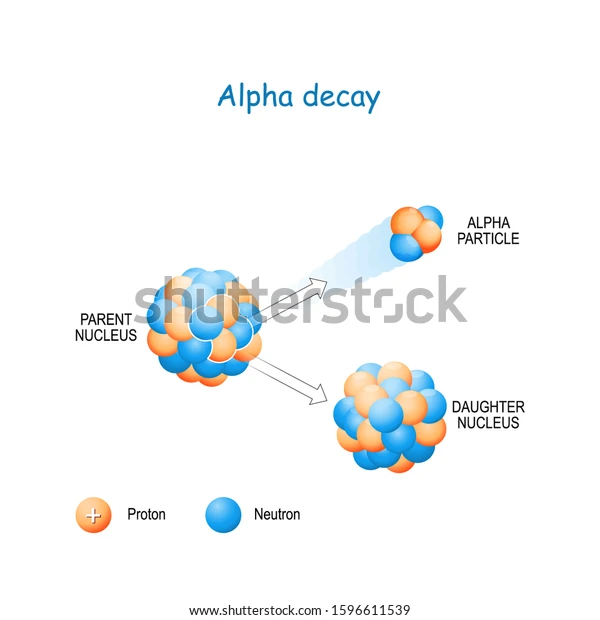
Alpha Decay
Since alpha decay involves the emission of nucleons, parent nucleus, which is the nucleus undergoing alpha decay, is changed. Its atomic and mass numbers decrease by 2 and 4, respectively. Simultaneously, energy is also released in the form of electromagnetic radiation, which is why the process is termed radioactive.
Alpha Decay Equation
It is possible to represent nuclear reactions via equations. The general equation of alpha decay is as follows:
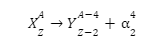
Here,
- X is the parent nucleus which undergoes alpha decay.
- Y is the daughter nucleus which the parent is converted into.
- α is the alpha particle that is emitted as a byproduct, along with energy.
Understanding Q Value of Alpha Decay
To understand the energy involved in a nuclear reaction and whether it is possible for the reaction to take place, Q value can be used. It is the difference in kinetic energies of initial and final nuclei and for alpha decay, we find it as follows: take the difference in the masses of the initial elements and the final products of the reaction, and multiply by \({c^2}\). That is,
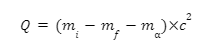
Here:
- Q is the Q value of the reaction.
- mi is the mass of the initial nucleus.
- \({m_f}\) is the mass of the final nucleus.
- \({m_α}\) is alpha particle mass.
- c represents the vacuum velocity of light.
It should be noted that units are important while working with such equations. The above equation is for the case where masses are given in units of MeV/\({c^2}\).
As previously mentioned, Q value can help us understand whether a reaction is possible. Not all elements can undergo all types of reactions. For example, whether alpha decay is possible depends on the Q value described above. Alpha decay is accompanied by a release of energy, which happens when the Q value is negative.
On the other hand, a very stable parent nucleus will have greater mass than that of the final products, making the Q value positive. This would correspond to energy input instead of output, making alpha decay in such a case, unfavourable. Thus, Q value can help us understand whether a reaction is possible or not.
What are the Major Components of the Equation that Represents Alpha Decay?
The alpha decay equation is as follows:
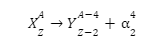
Its major components are summarized below:
- X represents the original or parent nucleus. Usually, this is an unstable element and it is observed that most elements undergoing alpha decay have mass numbers greater than 200.
- Y is the nucleus obtained after the reaction is complete. It has lower mass and atomic numbers than the parent and as represented, these numbers decrease by 4 and 2, respectively. It is generally more stable.
- α is the alpha particle. In simple words, alpha particles are just nuclei of Helium atom.
Alpha Decay Example
1. Decay of Radium-226
The decay of Radium-226 is a common example. Ra-226 is a very stable isotope with half-life of 1600 years but it can undergo alpha decay to generate Radon gas. The process also releases ionizing radiation.
The equation representing this decay is as follows:
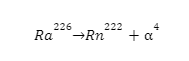
2. Decay of Uranium-238
The decay of Uranium 238 into Thorium is one of the most commonly cited alpha decay examples. Take a look:
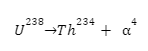
What happens in Alpha Decay?
We mentioned that the process of alpha decay is radioactive. That is, energy in the form of radiation is released. Further, alpha decay causes an unstable nucleus to change into a more stable one. This parent nucleus is generally heavy and when alpha decay occurs, it releases an alpha particle, which reduces its mass and makes it more stable. The alpha particle is ejected and travels a certain distance before becoming inert.
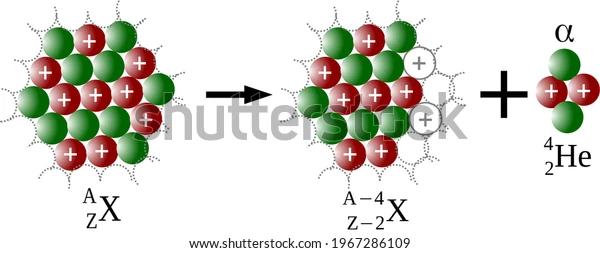
Helium nucleus emission
Gamow Theory of Alpha Decay
The energy of the alpha particle emitted can help us better understand the reaction. There are a large number of ways to measure this energy and a theoretical approach is to use Gamow’s theory, which connects the half-life of the process with the energy of the alpha particle. The foundation of this theory is in quantum mechanics and thus, its derivation is slightly complex.
The theory assumes the daughter nucleus and the alpha particle to be present inside the parent before the reaction occurs. Since nuclear potential is immensely strong, classical mechanics forbids these nuclei from escaping. However, quantum mechanically, the concept of “tunnelling” allows for a small probability of escape for these particles.
The relation derived from this hypothesis is the same as the one empirically derived by Geiger and Nuttal. It is summarized below:
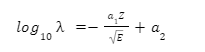
- λ is the decay constant.
- Z is the atomic number of the parent.
- E is the total kinetic energy of the daughter and the alpha particle.
- \({a_1}\) and \({a_2}\) are constants.
Summary
It is possible for certain elements in nature to be transformed into other elements via processes known as nuclear reactions. One common example of such reactions is nuclear decay, where an unstable nucleus decays into a more stable one by releasing energy and nucleons. Alpha decay is also a type of nuclear/radioactive decay, which occurs when an unstbale parent nucleus decays into a stabler one. In this process, an alpha particle is released, which is a Helium atom carrying a charge of +2e. The equation of alpha decay is given below:
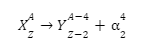
In alpha decay, mass and atomic numbers reduce by 4 and 2, respectively.
Gamow’s theory of alpha decay relates half-life of parent with the energy of alpha particle and uses quantum mechanical calculations to arrive at the result. It posits that the products of the reaction are present in the parent already and the decay occurs by quantum mechanical tunnelling.
Frequently Asked Questions
1. Are alpha particles dangerous?
Alpha particles can not penetrate the human skin too much and thus, are comparatively safe. But since our eyes are sensitive, alpha particles can damage the cornea. Further, if an alpha particle source enters the body, it can damage organs from inside.
2. How far do alpha particles penetrate into matter?
Alpha particles easily absorbed even by a thin sheet of paper and they become inert after a few cm of travelling in air.
3. What is the typical kinetic energy of an alpha particle?
Alpha particles travel are heavy and thus, travel at around 5 MeV. This is equivalent to a velocity of 15,000 km/s.
4. What other types of nuclear decay are observed?
Alpha, beta, and gamma decay are three common nuclear decays of which, gamma decay is the most dangerous one. It emits high energy photons that can penetrate human skin and cause cancer.
5. What are the uses of alpha decay processes?
Americium-241 is an alpha particle source that is used in smoke detectors. The process is also used in artificial pacemakers for generating power. Other medical applications include Radium-223 for the treatment of bone cancer.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


