What Are Aldehydes?
Carbon (C) and oxygen (O) form a double bond in aldehyde, making it a carbonyl functional group; carbon (H) and an alkyl group (C=O) form a single bond. As a rule of thumb, aldehyde is denoted as R-CHO. The letter “R” stands for the alkyl group. Carbon (C) is double-bonded to oxygen (O) in all aldehyde chemical compounds, forming the carbonyl group. The prefix “al” is appended to the name of any chemical compound that contains an aldehyde group
What Are Ketones?
A ketone also contains a carbonyl group and is thus a functional group. Here, two alkyl groups (R) are connected by a single bond while the carbonyl carbon (C) is double-bonded to oxygen (O). Ketones are typically written as R-CO-R, where R is an alkyl group. The prefix “one” is added to the name of any compound that contains a ketone group.
Properties of Ketones
To put it simply, ketones are liquids at room temperature. Use acetone as an illustration. Ketones have a higher boiling point than aldehydes and alcohols because the carbonyl group they contain exhibits dipolar attraction. The compound’s boiling point rises proportionally with its magnitude. In general, ketones of a lower molecular weight dissolve well in water, but this solubility declines with increasing ketone molecular size.
Properties of Aldehydes
Aldehydes come in a wide range of physical forms. Formaldehyde is one such chemical that, at room temperature, exists in the gaseous state. Formalin is the name given to an aqueous solution of the substance. Most other common aldehydes are liquids at room temperature, however others, depending on the number of alkyl groups, are solids. The lower aldehydes have a strong odour, whereas the higher aldehydes are pleasant, which is why they are used in fragrances.
Due to the dipolar attraction seen in the polar carbonyl group present in aldehyde, the boiling point of aldehyde is generally high.
Aldehyde show dissolution in water and other solvents. This is because they have lone pair of electrons in the oxygen (O) of the carbonyl group (C=O) which forms an H- bond with the hydrogen of water. The aldehydes which contain four carbon atoms are generally soluble in water but the solubility of aldehyde in water decreases as the size of the alkyl group increases.
Occurrence Of Ketones And Aldehydes
Many different types of microbes and plants are natural sources of ketones and aldehydes. Examples include cinnamaldehyde from cinnamon bark, citral from lemongrass, vanillin from vanilla bean, carvone from spearmint and caraway, camphor from the camphor tree, and helminthosporal from poisonous fungi, to name a few. Ketones can also be found in animals, such as the muscone found in musk deer, the sex hormones testosterone in males and progesterone in females, and the adrenal hormone cortisone.
Preparation Of Ketones And Aldehydes
Preparation of Aldehyde
Oxidation of primary alcohols: Primary alcohols can be treated with Pyridinium chlorochromate in dichloromethane to form aldehydes.
Dehydrogenation of primary alcohols : Dehydrogenation means the removal of the hydrogen molecule. Primary alcohol undergoes dehydrogenation in the availability of copper forms aldehyde.
From acid chlorides
The reduction of the acid chloride with hydrogen in the availability of palladium catalyst present over barium sulphate with a small quantity of quinoline gives aldehyde. The reaction is said to be Rosenmund’s reaction.
Preparation from carboxylic acid
When the vapours of carboxylic acid are passed over manganese oxide at 573 K, the aldehyde is produced. A common example is passing formic through manganese oxide at 573 K to give formaldehyde, carbon dioxide and water.
Preparation from alkenes
Alkenes reacting with ozone in presence of carbon tetrachloride give ozonides which further break down into two molecules of aldehyde in presence of zinc dust and water.
Preparation of ketones
From acid chloride: On reacting with dialkyl cadmium, ketone compound are formed from acid chloride.
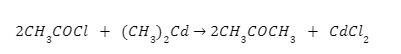
Here, acetyl chloride reacting with dimethyl cadmium forms acetone.
From carboxylic acid: When vapours of acetic acid is passed through manganous oxide, acetone is produced.
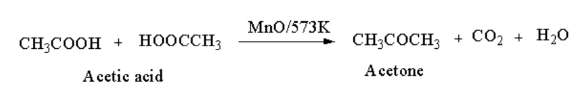
From alkenes:
2, 3-Dimethylbut-2-ene on ozonolysis gives acetone.
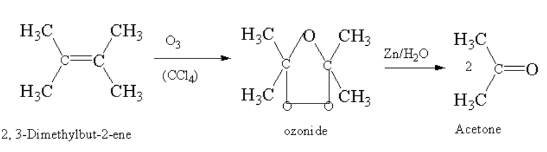
Here, 2, 3-Dimethylbut-2-ene reacting with ozone in presence of carbon tetrachloride gives ozonide which breaks down to acetone.
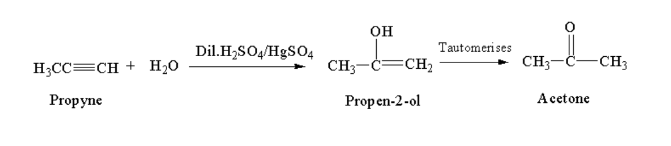
From akynes:
Propyne reacting with a hydrated mixture of dilute sulphuric acid and mercuric sulphate gives acetaldehyde.
Uses Of Aldehydes And Ketones
Perfumes typically include organic compounds containing aldehyde, such as vanilla, orange rind, rose, and citronella. Because of their pleasant aroma, these chemicals are synthesised in laboratories and used in the creation of perfumes and colognes. Because of its signature scent, benzaldehyde is also a popular ingredient in fragrances.
Aldehydes have several industrial applications, including in the production of chemical compounds, dyes, and resins. Because of its effectiveness as a preservative for biological specimens and its antimicrobial qualities, formaldehyde is often manufactured industrially on a larger scale. The most widely utilised ketone molecule, acetone is primarily employed as a solvent in the production of chemicals like iodoform and iodoform. The nail polish remover contains it.
Formation by Oxidation of Alcohols
Aldehyde: Primary alcohol oxidises of aldehyde in presence of acidified potassium dichromate (\({{\bf{K}}_2}{\bf{C}}{{\bf{r}}_2}{{\bf{O}}_7}\)) or alkaline potassium permanganate (\({\bf{KMn}}{{\bf{o}}_4}\)).
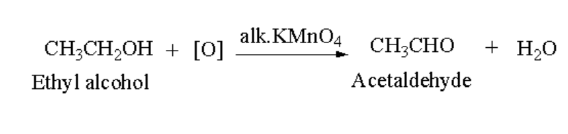
Here, ethyl alcohol oxidises to acetaldehyde.
Ketone: Secondary alcohol on oxidation in presence of acidified potassium dichromate (\({{\bf{K}}_2}{\bf{C}}{{\bf{r}}_2}{{\bf{O}}_7}\)) or alkaline potassium permanganate (\({\bf{KMn}}{{\bf{o}}_4}\)) gives ketone.
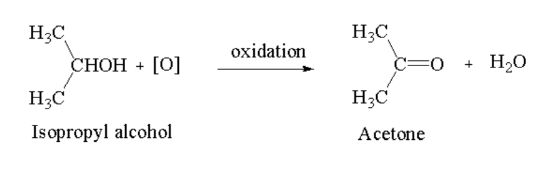
Here, isopropyl alcohol is oxidised to acetone.
Formation by Alcohols Dehydrogenation
Aldehyde: Primary alcohol undergoes dehydrogenation in the availability of copper forms aldehyde.
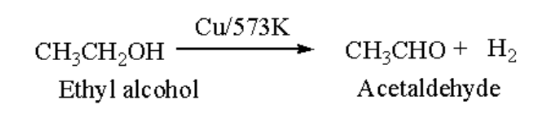
Ethyl alcohol on heating with copper changes to acetaldehyde and hydrogen molecule is removed.
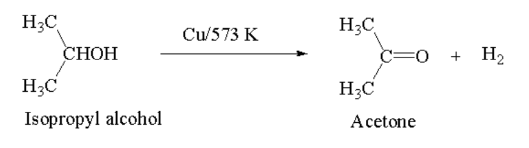
Ketone: Heating of secondary alcohol in presence of reduced Cu at 573 K forms ketone.
Summary
Carbonyl-containing organic substances include aldehydes and ketones. Aldehyde and ketones can be synthesised industrially on a massive scale in addition to being derived naturally from plants and bacteria. Its versatility in application has led to their widespread use. Both chloroform and iodoform require acetone as an input in their production. Dye manufacturing (like malachite green) and chemical synthesis (such benzoic acid and benzoyl chloride) all rely on benzaldehyde. There are a variety of additional organic molecules that can be used as building blocks for making aldehyde and ketones, respectively.
Frequently Asked Questions
1. Which test is common for the detection of both aldehyde and ketone groups?
Ans: Both aldehyde and ketone groups give 2, 4-Dinitrophenylhydrazine. When 2,4-DNP or Brady’s reagent is reacted with pure carbonyls, it forms precipitate which is yellow-orange in colour.
2. What is a Schiff Base?
Ans: A Schiff base is a compound with the general structure R2C=NR’ and is considered as a subclass of imines. Schiff’s test is a chemical test used to check for the presence of aldehydes in a given analyte.
3. Out of aldehydes and ketones which one is more reactive and why?
Ans: Aldehydes are more reactive towards nucleophilic addition reactions. Since the carbonyl group of ketones is connected to alkyl groups, and the alkyl group has electron donating inductive effect, it increases electron density on carbon of carbonyl group in ketones, making it less electrophilic.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


