Introduction
We currently know about 118 different chemical elements. These elements combine to form numerous compounds. These compounds are classified into Acids, Bases, and Salts based on their chemical properties. “All substances that produce \({H^ + }\) ions when dissolved in water are known as acids, while those that produce \(O{H^ – }\) ions when dissolved in water are known as bases.” When acids and bases are mixed together, they lose their acidic and basic properties, i.e. neutralise, and form salts.
What are Acids
“The word acid comes from the Latin word ‘acidus’ or ‘acere,’ which means sour. The most common feature is their sour taste. In its aqueous solution, an acid produces an ionizable hydronium ion (\({H_3}{O^ + }\)). It causes blue litmus paper to turn red. These dissociate in an aqueous solution to form their constituent ions, as illustrated by the examples below.”
\[HCl{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}{H^ + } + {\rm{ }}C{l^ – }\]
\[{H_2}S{O_4}\left( {aq} \right){\rm{ }} \to {\rm{ }}2{H^ + } + {\rm{ }}SO_4^{2 – }\]
\[C{H_3}COOH{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}{H^ + } + {\rm{ }}CH3CO{O^ – }\]
What are Bases
Bases are distinguished by their bitter taste and soapy texture. A base is a substance that produces the hydroxyl ion (\(O{H^ – }\)) in an aqueous solution. Bases cause red litmus paper to turn blue.
The bases dissociate in an aqueous solution to form their constituent ions, as shown in the examples below.
\[NaOH{\rm{ }}\left( {aq} \right){\rm{ }} \to {\rm{ }}N{a^ + } + {\rm{ }}O{H^ – }\]
\[Ca{\left( {OH} \right)_2} \to {\rm{ C}}{a^{2 + }} + {\rm{ }}2O{H^ – }\]
What are Salts
When an acidic solution is treated with an alkaline solution or aqueous solution of a metal oxide, a salt is formed, and the solution becomes neutral. A neutralization reaction occurs when \({H^ + }\) ions from an acid combine with \(O{H^ – }\) ions from the base of a metal oxide.
The chemical reactions shown below demonstrate the formation of salt.
\[HCl{\rm{ }} + {\rm{ }}NaOH{\rm{ }} \to {\rm{ }}NaCl{\rm{ }} + {\rm{ }}{H_2}O\]
\[{H_2}S{O_4} + Ca{\left( {OH} \right)_2}\, \to {\rm{ }}CaS{O_4} + {\rm{ }}2{H_2}O\]
Uses of Acids
- Sulphuric acid is used in the production of fertilizers such as ammonium sulphate, detergents, explosives, plastics, dyes, chemicals, etc.
- In the textile, food, and leather industries, hydrochloric acid is used as a dye. It is used to remove oxide films from steel objects prior to galvanization.
- Nitric acid is used in the production of fertilizers like ammonium nitrate, explosives like trinitrotoluene (TNT), plastics, and dye.
Uses of Bases
- Sodium hydroxide is commonly used in the production of soap, as well as synthetic fibre (Rayon) and paper.
- The reaction of calcium hydroxide, also known as slaked lime, produces bleaching powder.
- Magnesium Hydroxide, which acts as an antacid for the body, is used to neutralise excess acid in the stomach and cure indigestion.
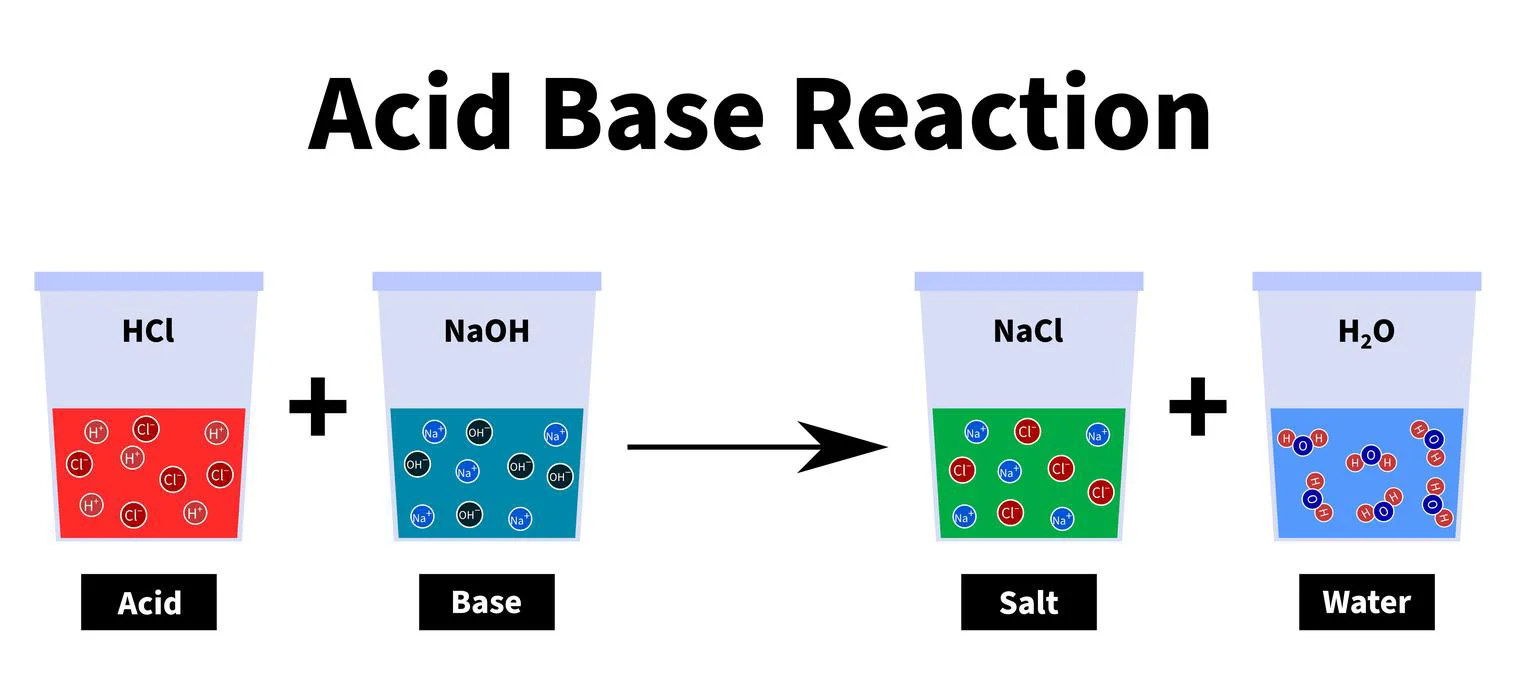
Summary
The compounds are classified into Acids, Bases, and Salts based on their chemical properties. “All substances that produce \({H^ + }\) ions when dissolved in water are known as acids, while those that produce \(O{H^ – }\) ions when dissolved in water are known as bases.” When acids and bases are mixed together, they lose their acidic and basic properties, i.e. neutralise, and form salts.
Frequently Asked Questions
1. What are the main differences between a strong acid and a weak acid?
| Strong Acids | Weak Acids |
| When exposed to water, strong acids completely dissociate into their ions. | In an aqueous solution, weak acids are molecules that partially dissociate into ions. |
| A strong acid solution has a very low pH. | A weak acid solution has a pH of 3-5. |
| It releases all the H+ ions into the solution. | Partially releases all H+ ions to enter the solution. |
2. What are the main differences between a strong base and a weak base?
| Strong Bases | Weak Bases |
| In a solution, a strong base can completely dissociate into its cation and hydroxyl ion. | A weak base partially dissociates into its hydroxyl ion and cation, resulting in an equilibrium state. |
3. Do acids conduct electricity?
Ans. The conductivity is due to the presence of ions. Acids dissociate to form (\({H^ + }\)) ions in solutions. Hence, acids conduct electricity.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


