Introduction
Ordinary substances like table salt are examples of binary compounds. Rock salt structure refers to the crystalline arrangement of salt. Sodium and chlorine ions are organised in a lattice pattern, which is made visible by this structure. Sodium and chlorine ions form a binary system in the sodium chloride lattice. It is essential to learn about these binary compound structures in order to comprehend the characteristics of various materials. Improved elasticity, strength, conductivity, and other physical qualities can be achieved through the synthesis and manufacturing of new materials in this branch of chemistry.
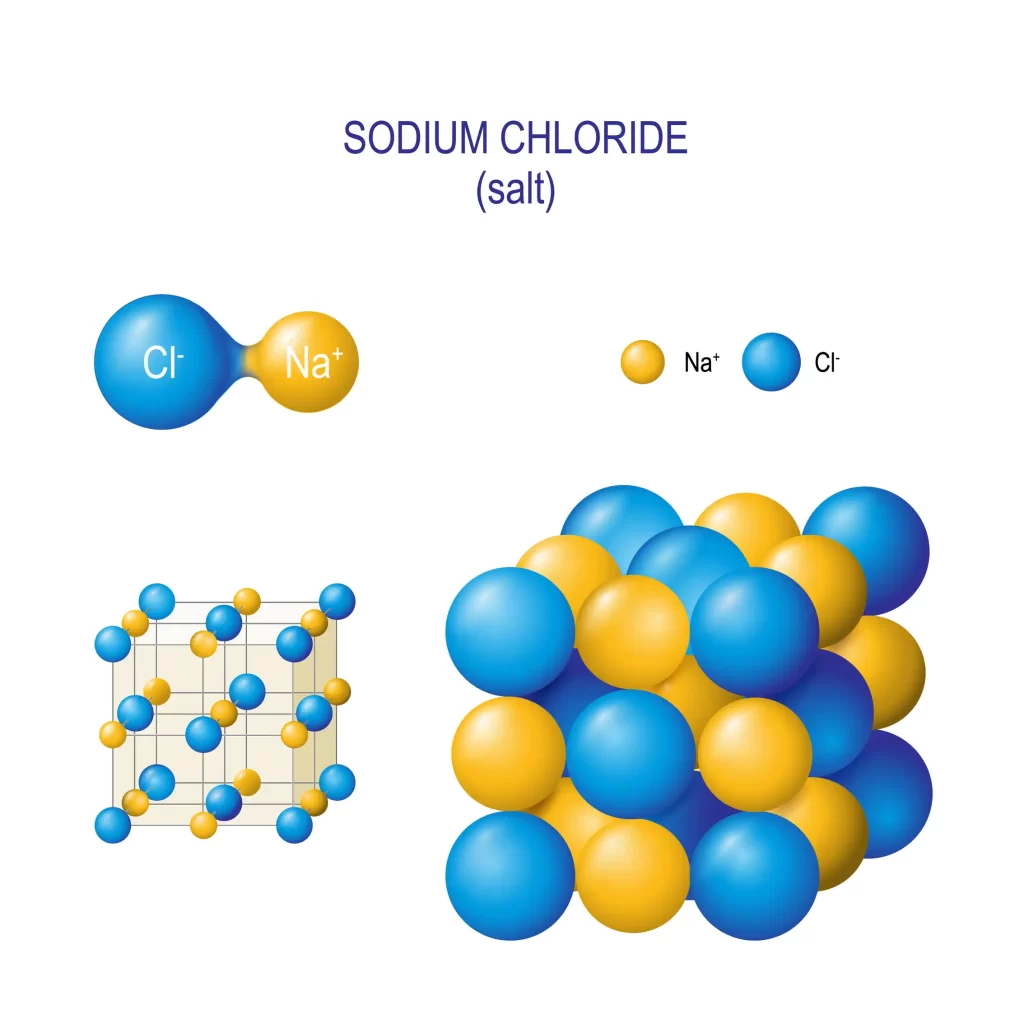
Structure of Sodium Chloride
What are Binary Compounds?
Binary compounds are those that consist of two distinct components combined together. Element refers to a material that cannot be chemically reduced to a simpler form. The simplest class of molecular compounds are the binary compounds. To further distinguish them, we might call them binary phase compounds or just binary phases. These compounds are crucial as they are used as building blocks in the production of several other key organic and inorganic chemicals. Water is an example of such a crucial substance. \({H_2}O\) consists of hydrogen and \({O_2}\), which are the two elements that may combine to form water. Water is a V-shaped covalent binary molecule. Two hydrogen atoms are covalently bonded to an oxygen atom with two lone pairs of electrons, making up a water molecule.
The Naming of Binary Compounds
Naming a binary compound is the same as naming any other kind of chemical. In the IUPAC system, you’ll find:
- To identify which cation is present in a binary compound, the cation’s name comes first.
- Binary compounds have their anion names written after the cation names.
- If you add the suffix -ide to the anion’s name, you get the element’s name. For instance, fluoride is the name for the fluorine anion.
- Among these, iron (II) oxide (FeO) is one example. Because iron’s oxidation state is +2, a roman number representing two is appended to the cation’s name.
- If there are more than two of each element in a binary complex, the ion count of each element is included in the ion’s name.
- Dinitrogen trioxide (\({N_2}{O_3}\)) is one such example. The prefix “di” is added to nitrogen because there are two of the element, while the prefix “tri” is given to oxide because there are three of the element’s oxygen atoms.
- When a binary compound contains a transition metal cation, the oxidation number of the cation is shown after the cation’s name in Roman numerals.
- Potassium chloride, for instance, is abbreviated as KCl. In this situation, potassium is a cation and chloride is an anion.
Examples of Binary Compounds
Covalent binary compounds-
Such compounds contain atoms of two elements joined by covalent bonds:\({N_2}O\) ,\(CC{l_4}\) ,\({H_2}{O_2}\), \(C{H_4}\), etc.
Ionic binary compounds –
Such compounds contain ionic bonds that link atoms of two elements : NaCl, MgO, NaBr,\(BaC{l_2}\), etc.
Transition metal binary compounds-
AuI, \(FeC{l_2}\)2, CuI, Lead (II) fluoride, \(PbC{l_2}\)2, etc.
Binary Acids
Binary acids, also called hydracids, consist of hydrogen and a non-metallic element. There are two types of acids: oxyacids and binary acids. Oxyacids are compounds made up of hydrogen, oxygen, and sometimes additional elements as well. The prefix “hydro,” followed by the element name, and then the suffix “ic” are used to name binary acids. Hydrochloric acid (HCl) is one such chemical. The primary building blocks of hydracids are hydrogen and halogens. Many parameters, including the bond energy of bonding between anion and hydrogen, the solvation energy of anion, anion electron affinity, etc., determine the potency of these acids. The hydrogen-ion connection is weaker in acids because hydrogen is a more electronegative element.
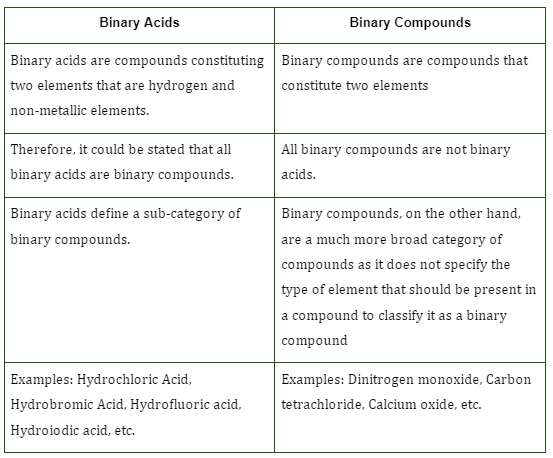
Difference between Binary Acids and Binary Compounds
Binary Ionic Compound
Binary ionic compounds are those that consist of atoms of two different elements bound together by electrostatic repulsion. For example NaCl, \(BaC{l_2}\), etc. These compounds form conduction solutions because of free moving ions.
Summary
In chemistry, the fundamental building blocks are binary compounds. Learning about larger molecules and compounds is easier after establishing a firm grasp of these simpler ones. There is a large variety of uses for these chemicals since this class of compounds is so vast. For example, a binary compound might be a binary covalent compound, a binary ionic compound, a binary acid, a binary transition metal compound, etc. Pharmacy,organic chemistry, analytical chemistry, industrial chemistry, materials chemistry, biotechnology, polymer chemistry, etc., are just some of the many scientific disciplines that study these compounds and their properties.
Frequently Asked Questions
1. Write the order of increasing acidity of hydrohalo acids.
The order of increasing acidity is:
HI>HBr>HCl>HFh
This happens because as the size of the anion increases, its nucleophilicity increases. I– is a strong nucleophile and prefers to stay in such a state. Thus its acidity is highest.
2. Are all binary compounds conducting in nature?
No, only ionic compounds are conducting in nature. This is because of the formation of free flowing ions which conduct charge. Binary covalent compounds are not conducting because their atoms are bonded.
3. Is rust a binary compound?
The chemical formula for rust is FeO. Thus, it is an example of a binary compound since it is only made of two types of atoms. It is an ionic binary compound with iron in +2 oxidation state and it has an octahedral geometry.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


