Introduction
Acids can take lone pairs of electrons from other substances, ions, or molecules. Whereas, Bases are those substances, ions, or molecules that can transfer lone pairs of electrons, whereas based on specific experimental findings, chemical substances were initially categorized as bases and acid substances were initially categorized as bases and acids based on specific experimental outcomes. The Arrhenius concept, the Bronsted-Lowry concept, the solvent system concept, the Lux-flood concept, and the Lewis concept are some of the more well-known contemporary acid-base concepts.
Acid Strength
The ability of an acid to separate into hydrogen ions (\({H^ + }\)) and anions in an aqueous solution are known as its acidic strength. It is represented by the HA chemical formula. The strongest bases are located at the top right of the graph, and the strongest acids are located at the top left. A strong base’s conjugate acid is a weak acid because a strong acid’s conjugate base is weak.
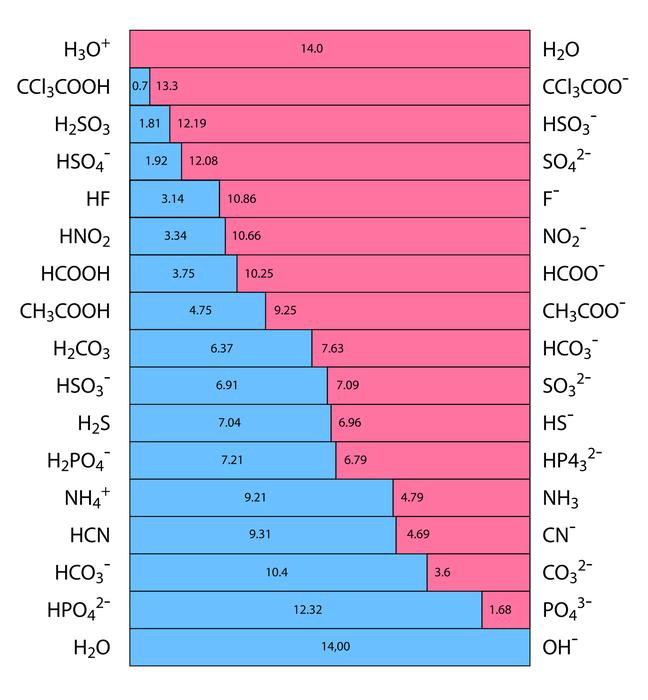
Calculating acid strength with the aid of the equilibrium constant (\({K_a}\)). As a result, chemicals like sulphuric acid \({H_2}S{O_4}\), nitric acid \(HN{O_3}\), and hydrochloric acid HCl are acidic.
\[HA \leftrightarrow {H^ + } + \;{A^ – }\]
For example,
\[HCl{\rm{ }}\left( {aq} \right){\rm{ }} + {H_2}O\left( l \right) \leftrightarrow {H_3}{O^ + }\left( {aq} \right) + \;C{l^ – }\left( {aq} \right)\]
Here, HCl has a greater tendency to lose a proton and therefore, equilibrium shifts more towards the right.
\({K_a} = \frac{{\Pr oducts}}{{{\mathop{\rm Re}\nolimits} ac\tan ts}} = \frac{{[{H_3}{O^ + }][C{l^ – }]}}{{[HCl][{H_2}O]}}\)
Accordingly, \({K_a}\) is used to describe the strength of only those acids that are weaker than \({H_3}{O^ + }\), and Kb is used to describe the strength of only those bases that are weaker than \(O{H^ – }\). Acidity is represented by the constant \({K_a}\).
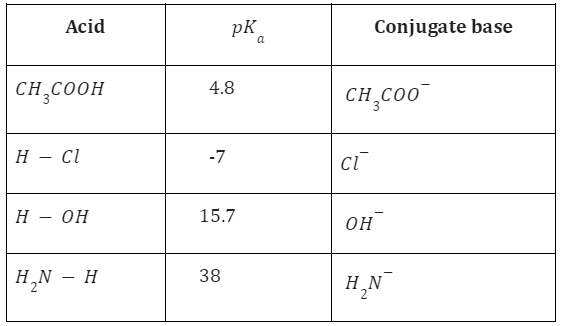
The term “\(p{K_a}\) value” is sometimes used to define an element’s acidity. The dissociation constant’s negative logarithm is known as the \(p{K_a}\) value.
\(p{K_a} = {\rm{ }} – log{\rm{ }}{K_a}\,and\,p{K_b} = – \log {K_b}\)
Strong and Weak Acids
Acids that have a great ability to donate protons are referred to as strong acids. Similar to this, weak acids are those acids, like acetic acid and carbonic acid, that have a high tendency to receive protons. The susceptibility of the base to accept its proton often determines the acid’s strength.
\[{\bf{C}}{{\bf{H}}_{\bf{3}}}{\bf{COOH}}{\rm{ }} + {\bf{N}}{{\bf{H}}_{\bf{3}}} \to {\bf{C}}{{\bf{H}}_{\bf{3}}}{\bf{CO}}{{\bf{O}}^ – } + {\bf{N}}{{\bf{H}}^{{\bf{4}} + }}\]
\[{\bf{C}}{{\bf{H}}_{\bf{3}}}{\bf{COOH}}{\rm{ }} + {{\bf{H}}_{\bf{2}}}{\bf{O}} \to {\bf{C}}{{\bf{H}}_{\bf{3}}}{\bf{CO}}{{\bf{O}}^ – } + {{\bf{H}}_{\bf{3}}}{{\bf{O}}^{ + \;}}\;\]
Acetic acid behaves as a strong acid with ammonia in this reaction, but as a weak acid in water. Hydrochloric acid (HCl), Nitric acid HNO3 and Sulphuric acid H2SO4 are examples of strong acids because they dissociate into ions completely in water.
Acid Strength Determining Factors
The relative conjugate base of acid also affects its strength. On the other hand, the weak conjugate base makes up the strong acid. The weak acid consists of a strong conjugate base.
- Effect of hybridization: Electronegativity and the s-character of the atom both impact how acidic the molecule is. The conjugate base will be more stable the more s-character there are in the hybrid orbitals.
- Periodic trends: The second row of the periodic table shows an increase in acid strength as we move from left to right because the acid gets stronger as the conjugate base gets weaker.
- Resonance effect: When the conjugate base is resonance stabilized, the acid strength rises.
- Inductive effect: The pull of electron density across an atom’s bonds was defined by the indicative effect. The more electronegativity, the stronger the effect; they are exactly proportional to one another.
Order of Acid Strength
The charge density determines an atom’s protons-accepting tendency (or electron pair donation) if there is a significant difference in the size of the atoms that receive the proton. The proton is more strongly drawn to a stronger negative charge.
Comparing the basic strengths of each conjugate base makes it relatively simple to compare the acidity of the bases. The basic strength of halides, for instance, varies as F>Cr> Br>I. This clarifies the order of conjugate acid strength, which is HF <HCL <HBr <HI.
Factors Affecting Acid Strength
The following factors influence an element’s acid strength:
- The polarity of bonds The acid strength of the H-A bond depends on its polarity. The proton tends to leave the molecule more readily when the link is extremely polar, making the molecule a strong acid.
- Bond strength- It is based on how strong the H-A bond is. The energy needed to break a bond decreases with bond strength. As a result, acids are stronger. When comparing the acid strengths of elements belonging to the same group in the periodic table, however, bond strength becomes more significant.
Summary
The information above has given you a thorough understanding of the strength of acid and base. It is found that the basicity or acidity of a chemical is greatly influenced by the inductive effects and charge delocalization. The types of bonds that an ion forms have a significant impact on the acid-base strength of a molecule. The possibility that \({H^ + }\) ions may dissociate increases with the strength of the bonding between protons and anions. In addition, the dissociation of \({H^ + }\) is enhanced by any factor that helps to stabilize the lone pair on the conjugate base.
Frequently Asked Questions
1. What is meant by Dissociation?
Ans: Chemical compounds with ionic connections, such as salts, can be divided into simpler compounds like ions, radicals, or atoms by a process called dissociation.
For example,
\(C{H_3}COOH\) dissociated into ions as:
\[C{H_3}COOH\left( {aq} \right) \leftrightarrow C{H_3}CO{O^ – }\left( {aq} \right){\rm{ }} + {H^ + }\left( {aq} \right)\]
2. Which acid is thought to be the weakest one in the world?
Ans: Those elements that have a low tendency to donate protons due to their high negative charge and which attracts protons more strongly are considered the weakest acid. Hence, hydrogen fluoride (HF) is considered the world’s weakest acid.
3. Even though acetic acid is very soluble in water, why is it characterized as a weak electrolyte?
Ans: The electrolyte’s strength is determined by how ionized it is in solution, not by its concentration. Due to its minimal ionization, acetic acid is a weak acid. It is more soluble because of a hydrogen connection between it and water.
 Mission Statement
Mission Statement
“Empower every student to achieve full potential”
88Guru has been established with the social objective of making quality video-based learning material available to all Indian students. Technology, Connectivity and Social Media are rapidly changing the world of Education and we wish to lead the transformation of the tuition industry in India.
88Guru is the perfect complement to the current tuition model. 88Guru creates a wonderful opportunity for children and parents to bond while engaging in a valuable learning activity. It also provides the complete curriculum at your fingertips for those moments when you need some help at short notice. We believe that this mode of tuition could be transformational, adding hours to a child's day while providing complete control over the learning process.
Every course is taught by the best teachers from India's top schools and conducted in an engaging manner to keep students involved. The e-learning process consists of video-based instructions, computer-graded assignments, and a dashboard which allows the student and parent to track progress.


